CDC announced Rocky Mountain spotted fever in people traveling to the U.S. from Tecate, Mexico
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…
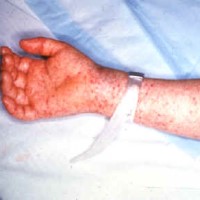
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…
On Nov. 27, 2023, University of Wisconsin-Madison researchers released a study that showed COVID-19 caused an alarming surge…
On Oct. 30, 2023, the California Department of Public Health (CDPH) reported the first locally acquired case of…
On Oct. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $42.1 million to fund various translational…

On Oct. 7, 2023, California became the first state to ban four chemicals used in well-known candies and…

On Oct. 2, 2023, the California Institute for Regenerative Medicine (CIRM) announced its approval of a new program…

On Sept. 29, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $43.8 million to fund projects aimed…
On Sept. 25, 2023, researchers at the University of California, Berkeley reported that engineered viruses also can generate…

On Aug. 27, 2003, The Institute for Collaborative Biotechnologies (ICB) announced it was funding a $50 million five…
On Aug. 24, 2023, NIAID reported that emerging resistance to common malaria treatments in Uganda could be connected…

On Aug. 24, 2023, researchers at City of Hope announced were awarded $32.3 million from the California Institute…
On Aug. 23. 2023, researchers co-led by University of California, Santa Cruz Assistant Professor of Biomolecular Engineering Karen…

On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…

On May 11, 2023, the U.S. Departments of Agriculture Animal (USDA) and Plant Health Inspection Service (APHIS) announced…

On Apr. 7, 2023, the U.S. National Park Service reported Highly Pathogenic Avian Influenza (HPAI) had been confirmed…

On Mar. 27. 2023, Thermo Fisher Scientific and the University of California, San Francisco, announced they will accelerate…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Oct. 26, 2022, the NIH announced it had awarded the University of Southern California (USC) $11.7 million…

On Oct. 5, 2022, researchers at UC Berkeley reported that coccidioidomycosisラalso known as Valley feverラan infectious disease, was…

On Sept. 20, 2022, the University of California, Davis announced that the amphibian decline in Latin America in…
On Apr. 22, 2022, Stanford University announced it was awarded a $75 million gift from Nike co-founder Phil…

On Mar. 20, 2022, the American Chemical Society awarded The Priestley Medal to Peter B. Dervan “to recognize…

On Mar. 10, 2022, schools with mandatory masking during the Delta surge had approximately 72% fewer cases of…

On Mar. 10, 2022, the City of Hope announced that its COVID-19 investigational vaccine licensed to GeoVax Labs…

On Mar. 4, 2022, Oxitec announced the publication by the U.S. Environmental Protection Agency of its approval to…

On Mar. 2, 2022, Sorrento Therapeutics announced that it had received clearance from the FDA for its investigational…

On Feb. 28, 2022, the Patent and Trial Appeal Board of the U.S. Patent and Trademark Office (USPTO)…

On Dec. 1, 2021, the California and San Francisco Departments of Public Health confirmed that a recent case…