COVID vaccines not linked to seizures
On Apr. 30, 2024, a meta-analysis of six randomized controlled trials in JAMA Neurology found no increase in seizures in…

On Apr. 30, 2024, a meta-analysis of six randomized controlled trials in JAMA Neurology found no increase in seizures in…
On Apr. 30, 2024, National Institutes of Health (NIH) researchers and collaborators announced they had discovered over 100…
On Apr. 29, 2024, Oregon Health Sciences University (OHSU) reported a patient with the first documented case of…
On Apr. 26, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding nearly $31…
On Apr. 26, 2024, an international team of researchers led by the National Cancer Institute (NCI) released an…
On Apr. 26, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BEQVEZ™ (fidanacogene…
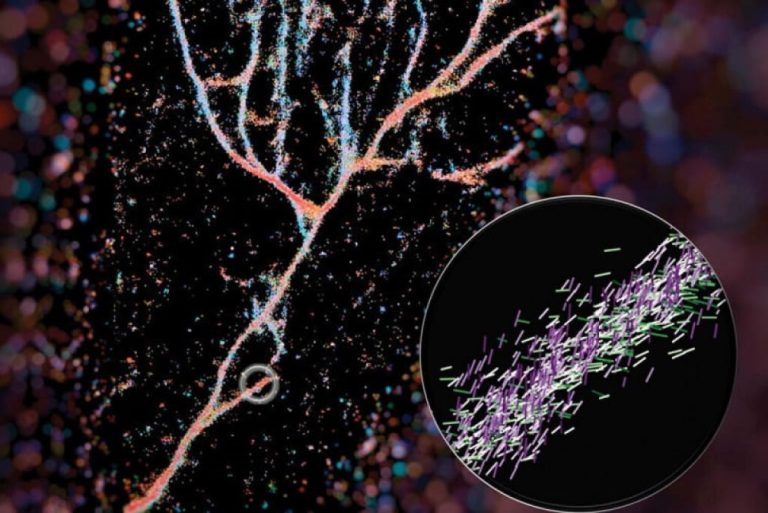
On Apr. 26, 2024, engineers at Washington University in St. Louis announced a new imaging technique that give…

On Apr. 26, 2024, the Colorado Dept. of Agriculture reported that the U.S. Department of Agriculture’s (USDA) National…
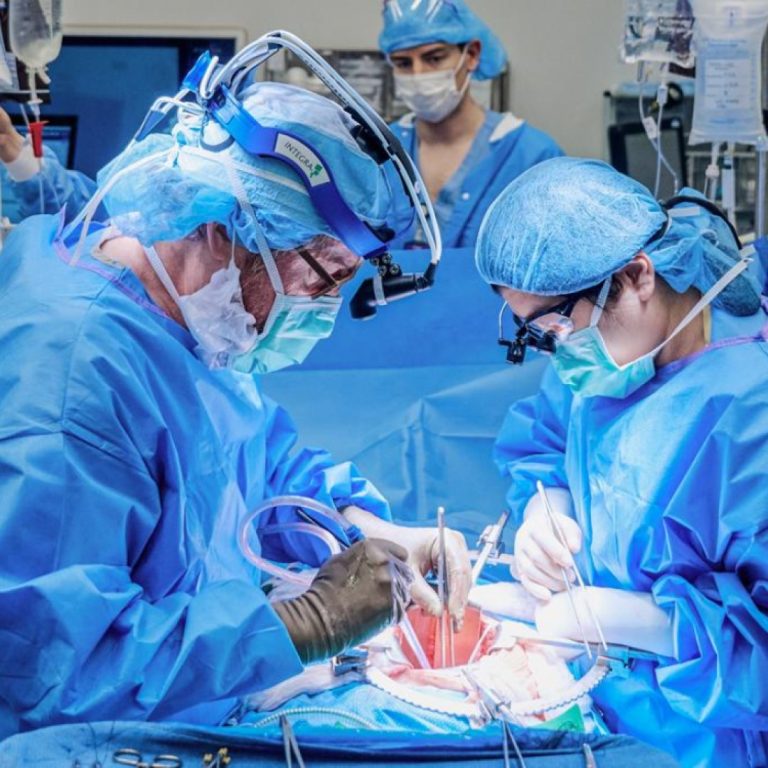
On Apr. 24, 2024, surgeons at NYU Langone Health announced they had performed the first combined mechanical heart…
On Apr. 23, 2024, the U.S. Food and Drug Administration (FDA) announced that nearly all (99%) of the…

On Apr. 22, 2024, Moderna announced a contract with the Ministry of Health in Brazil (Ministerio da Saude)…

On Apr. 21, 2024, United States Department of Agriculture’s (USDA) APHIS National Veterinary Services Laboratories announced it had…

On Apr. 15, 2024, scientists at University of California Riverside demonstrated a new, RNA-based vaccine strategy that is…
On Apr. 12, 2024, Oregon Health Sciences University (OHSU) announced research that shows using live SARS-CoV-2 virus revealed…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…

On Apr. 11, 2024, a study of data from more than 2 million children in Sweden reported that…
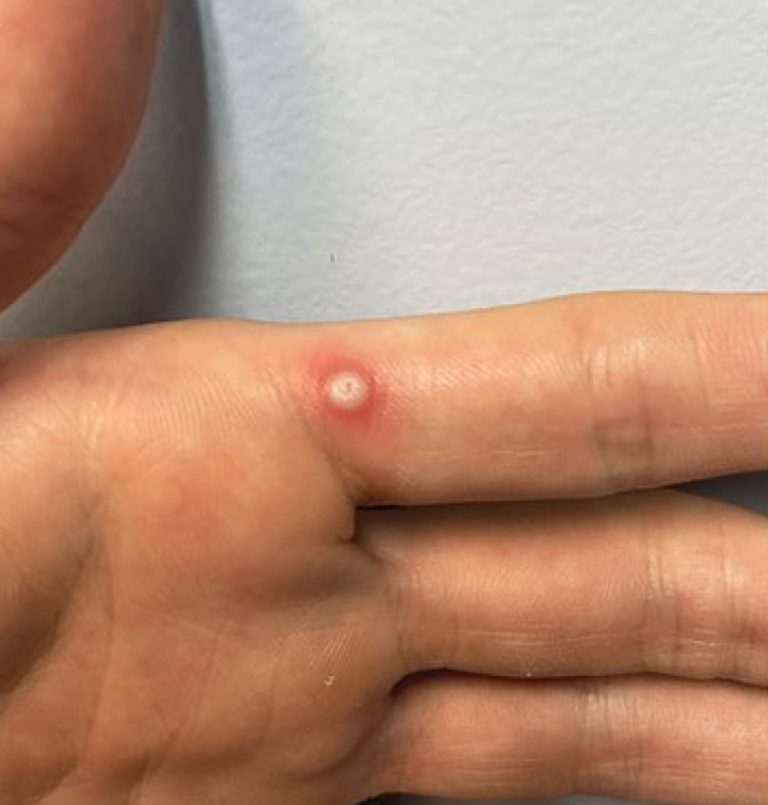
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On Apr. 10, 2024, researchers at the National Institutes of Health (NIH) applied artificial intelligence (AI) to a…

On Apr. 7, 2024, Edwards Lifesciences announced the release of data from the HUDDLE study that examined the…

On Apr. 5, 2024, groundbreaking was held for the Roy Blunt Soil Testing and Research Laboratory at the…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Apr. 2, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Apr. 1, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Apr. 1, 2024, Otsuka Pharmaceutica and Click Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…

On Mar. 29, 2024, the California Institute for Regenerative Medicine (CIRM) announced awards that support 11 projects in…

On Mar. 28, 2024, the American Chemical Society awarded The Priestley Medal to Carolyn R. Bertozzi “to recognize…

On Mar. 28, 2024, the Idaho State Department of Agriculture (ISDA) announced it had identified a highly pathogenic…
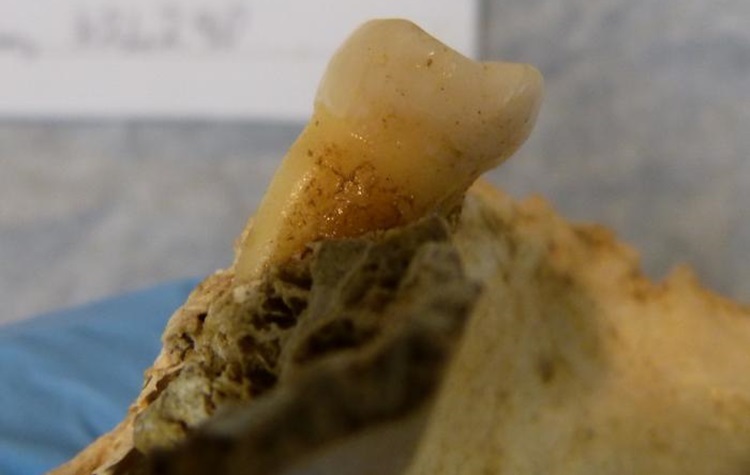
On Mar. 27, 2024, researchers from Trinity College Dublin announced they had recovered remarkably preserved microbiomes from two…