The CDC received FDA approval for a highly sensitive influenza PCR assay
In 2008, the U.S. Centers for Disease Control and Prevention (CDC) received U.S. Food and Drug Administration (FDA)…

In 2008, the U.S. Centers for Disease Control and Prevention (CDC) received U.S. Food and Drug Administration (FDA)…

In 2008, the Influenza Reagent Resource (IRR) was established by the Centers for Disease Control and Prevention (CDC)…

In 2008, the University of Missouri Research Animal Diagnostic Laboratory opened its new $15.5 million facility at Discovery…

On Dec. 19, 2007, the Human Microbiome Project (HMP), a $150 million initiative, was established by the National…

On Oct. 19, 2007, the U.S. Centers for Disease Control and Prevention (CDC) published updated recommendations for prevention…

On Sept. 28, 2007, the U.S. Food and Drug Administration (FDA) approved an Australian-made influenza vaccine called Afluria…

On Jun. 7, 2007, years before the COVID-19 pandemic swept across the globe, a group of about 100…

On Jan. 9, 2007, the Executive Committee of the Council of State and Territorial Epidemiologists approved an interim…

In 2007, the World Health Organization (WHO) reported the first significant outbreaks of Zika virus infection involving more…

In 2007, researchers at Livermore National Laboratory announced they had developed candidate signatures for pathogens that might be…
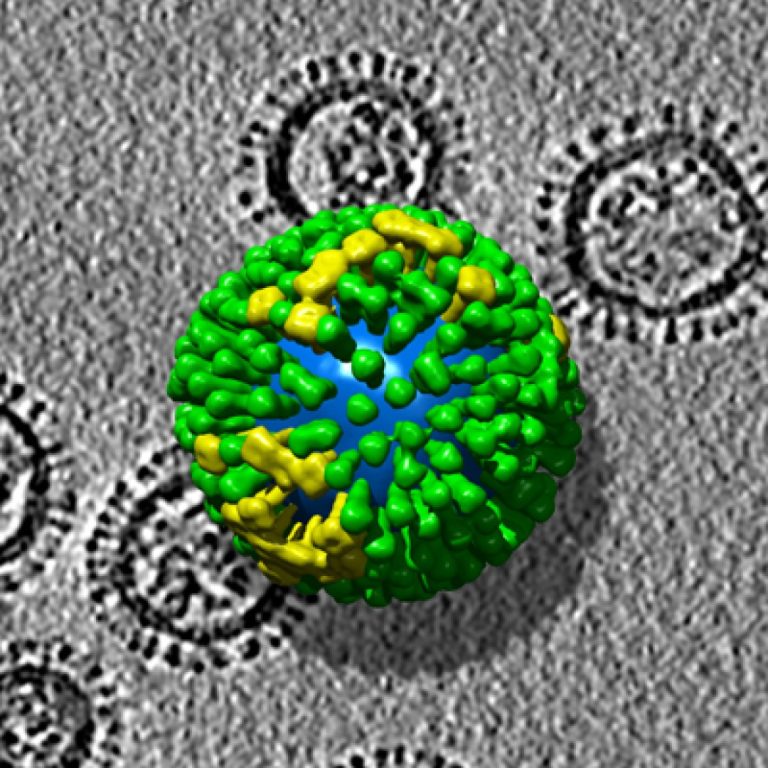
On Jan. 17, 2006, the Centers for Disease Control and Prevention (CDC) announced that it had stopped recommending…
On Jan. 6, 2006, the U.S. Centers for Disease Control and Prevention’s (CDC) advisory Committee on Immunization Practices…
On Dec. 23, 2005, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
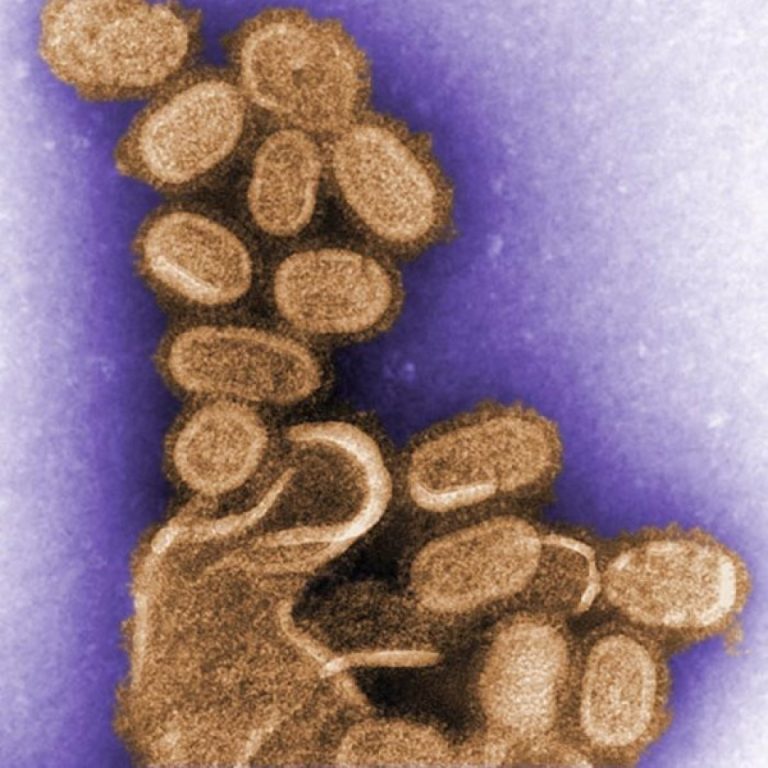
On Oct. 7, 2005, Jeffery Taubenberger, AH Reid, AE Krafft, Karen Bijwaard and Thomas Fanning published a report…
On Mar. 21, 2005, the U.S. Centers for Disease Control and Prevention (CDC) announced that a major public…

On Sept. 30, 2003, University of Iowa (UI) Microbiology Professor Mark Stinski made the discoveries of CMV promoter…

On Sept. 14, 2004, researchers at OHSU and OHSU’s Vaccine and Gene Therapy Institute announced a collaboration with…

On May 27, 2004, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had awarded contracts…

On Jul. 8, 2003, the U.S. Centers for Disease Control and Prevention (CDC) responded to U.S. outbreak of…

On Jun. 17, 2003, the first nasally administered influenza vaccine (FluMist by MedImmune) was licensed. This live influenza…

On Jan. 26, 2003, President George W. Bush, in his State of the Union Address, announced the Emergency…

In 2003, public health officials reported the re-emergence of H5N1 avian influenza for the first time since an…

On Dec. 3, 2002, Genentech drug Pegasys (peginterferon alfa-2a) was approved by the Food and Drug Administration (FDA)…

On Dec. 12, 2001, the World Health Organization (WHO) confirmed an outbreak of Ebola in Gabon. The WHO…
On Nov. 28, 2001, researchers discovered the E6 and E7 gene in HPV virus, linking cervical cancer cell…
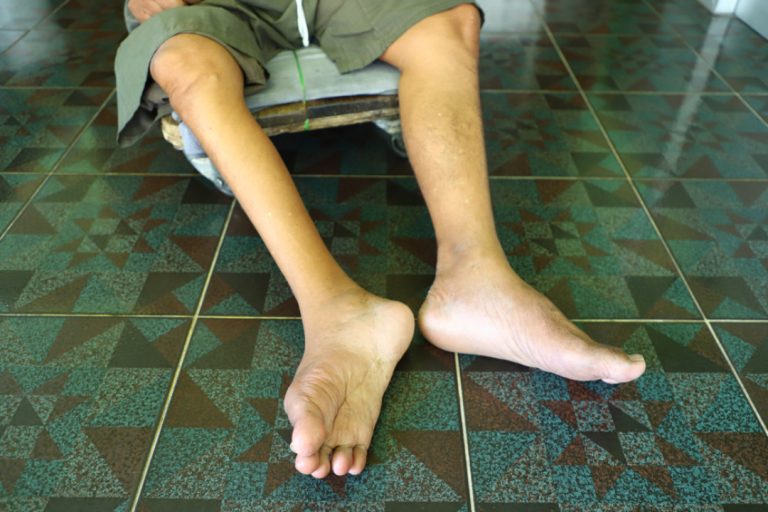
On Oct. 29, 2000, the Regional Commission for the Certification of Poliomyelitis Eradication certified that the Western Pacific…

On Aug. 22, 2000, researchers in Kenya announced they had field-tested twelve lines of sweetpotato variety CPT 560…

On Oct. 22, 1999, the Advisory Committee on Immunization Practices (ACIP), after a review of scientific data from…
In August 1999, an outbreak of encephalitis caused by West Nile virus (WNV) was detected in New York…

On Jun. 17, 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP)…