Emergent BioSolutions Receives New Contract for ACAM2000®, (Smallpox and Mpox (Vaccinia) Vaccine, Live) from the U.S. Government
On Sept. 9, 2025, Emergent BioSolutions announced that a contract modification has been executed in the amount of…

On Sept. 9, 2025, Emergent BioSolutions announced that a contract modification has been executed in the amount of…

On Jan. 2, 2025, SIGA Technologies announced that its antiviral treatment TEPOXX (tecovirimat 200 mg capsules), marketed as…
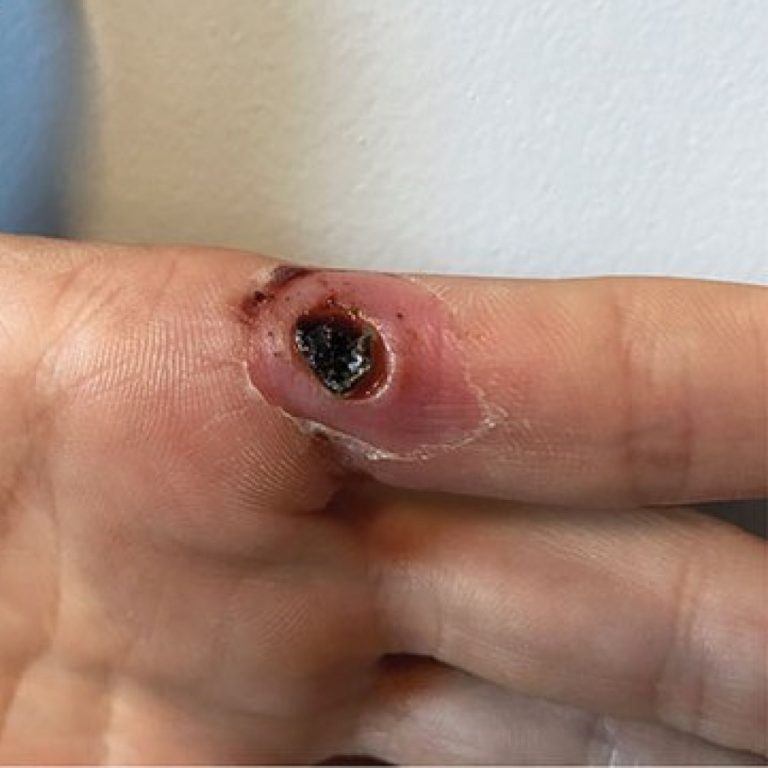
On Aug. 29, 2024, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…
On Jul. 2, 2024, Emergent BioSolutions announced it had received more than $250 millionin contract modifications from the…
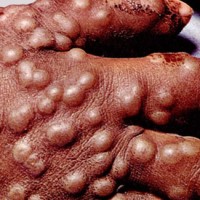
On Mar. 26, 2024, the National Academies of Sciences, Engineering, and Medicine released a report that said action…

On Jul. 27, 2023, SIGA Technologies announced that the U.S. Department of Health and Human Services exercised procurement…
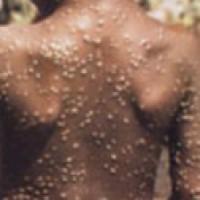
On Feb. 14, 2023, scientists from the National Institute of Allergy and Infectious Diseases announced they had developed…

On Aug. 29, 2022, the U.S. Department of Health and Human Services (HHS) announced it had provided approximately…

On Aug. 9, 2022, the U.S. Food and Drug Administration issued an emergency use authorization for the JYNNEOS…

On Jul. 6, 2022, LabCorp announced that it had begun testing for monkeypox using the U.S. Centers for…
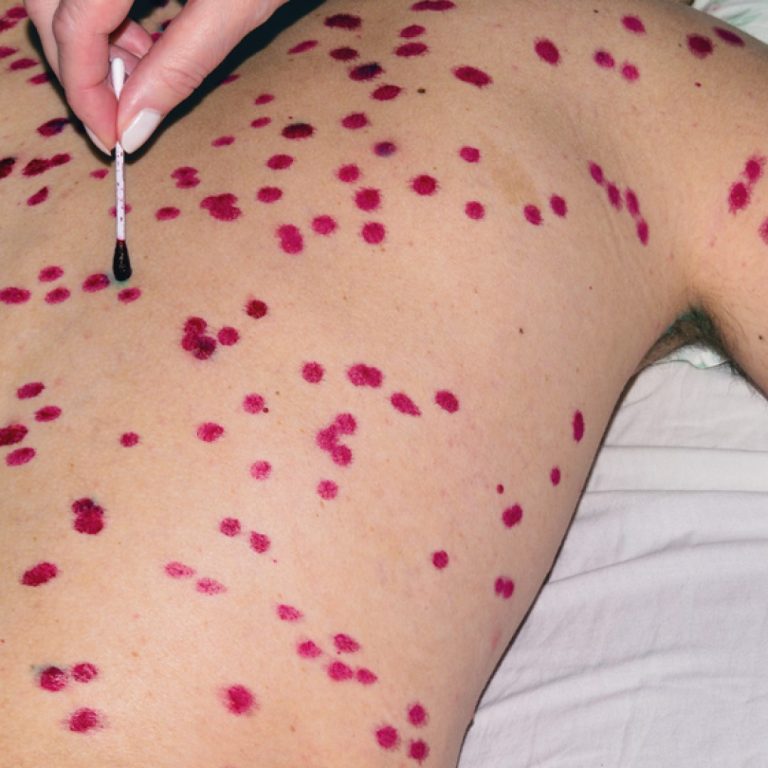
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…
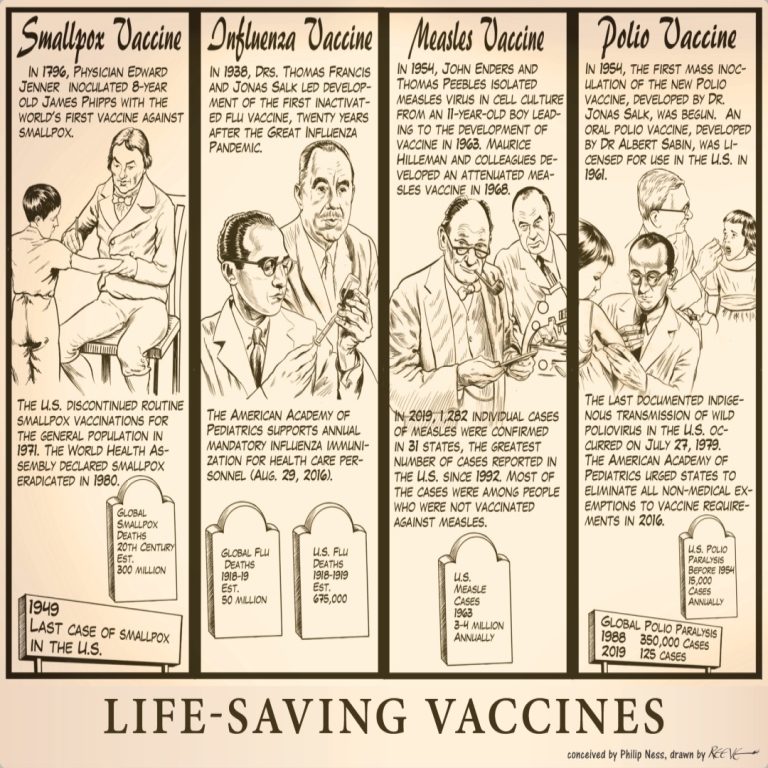
Our Life-Saving Vaccines cartoon illustrates four invaluable vaccines that have affected human civilization throughout history from smallpox and…
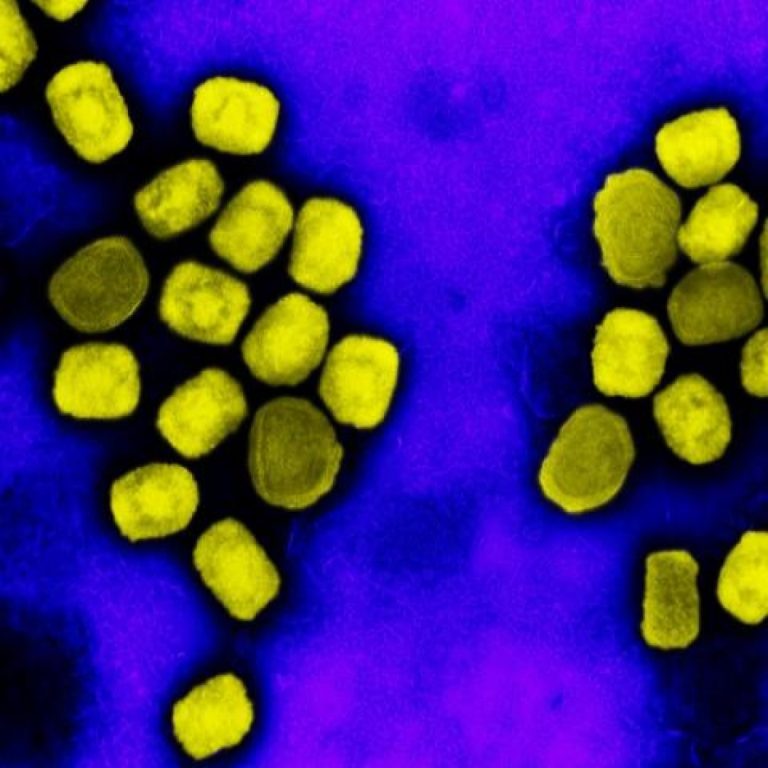
On Jul. 29, 2021, SIGA Technologies announced that it had entered into a collaboration with Oxford University in…
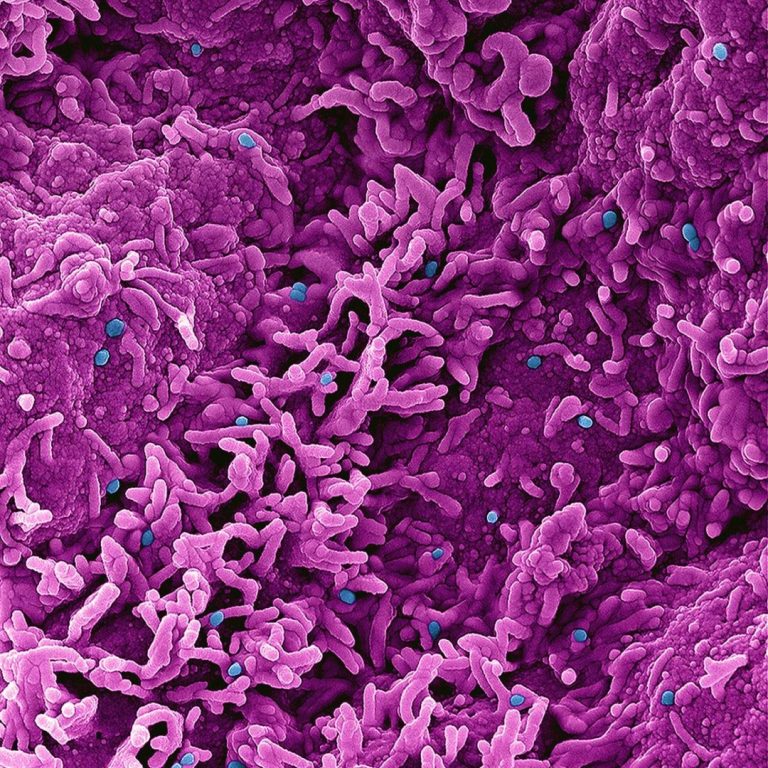
On Jun. 30, 2021, SIGA Technologies announced that it had provided TPOXX (tecovirimat) to Liverpool University Hospitals NHS…

On Jun. 4, 2021, Chimerix announced that the U.S. Food and Drug Administration (FDA) had granted TEMBEXAᆴ (brincidofovir)…

On Jan. 13, 2021, SIGA Technologies announced that the Public Health Agency of Canada had awarded a contract…

On Dec. 7, 2020, Chimerix announced that the FDA has accepted the filing of a New Drug Application…

On Oct. 8, 2020, SIGA Technologies announced that the Public Health Agency of Canada (PHAC) has issued an…

On Aug. 25, 2020, the independent Africa Regional Certification Commission (ARCC) for Polio Eradication officially declared that the…
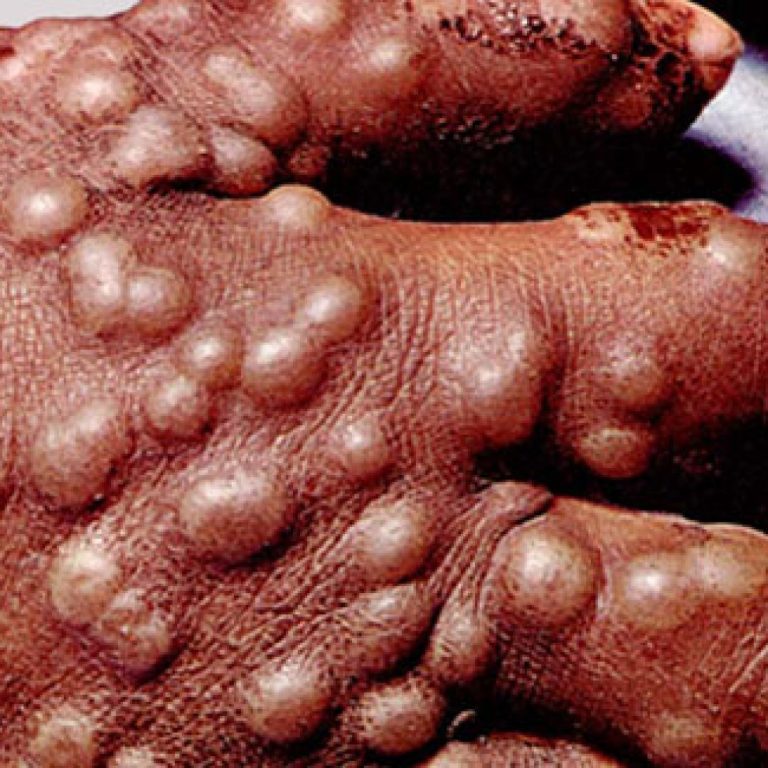
On Jun. 25, 2020, SIGA Technologies announced the deliveries of oral TPOXX (tecovirimat) to the U.S. Department of…

On Jun. 15, 2020, SIGA Technologies announced the U.S. Department of Defense (DoD) increased research and development funding…

On Jun. 1, 2020, SIGA Technologies announced its first international delivery of TPOXX (tecovirimat), with 2,500 courses delivered…

On Apr. 28, 2020, Chimerix announced it had received clearance from the FDA for a rolling submission of…
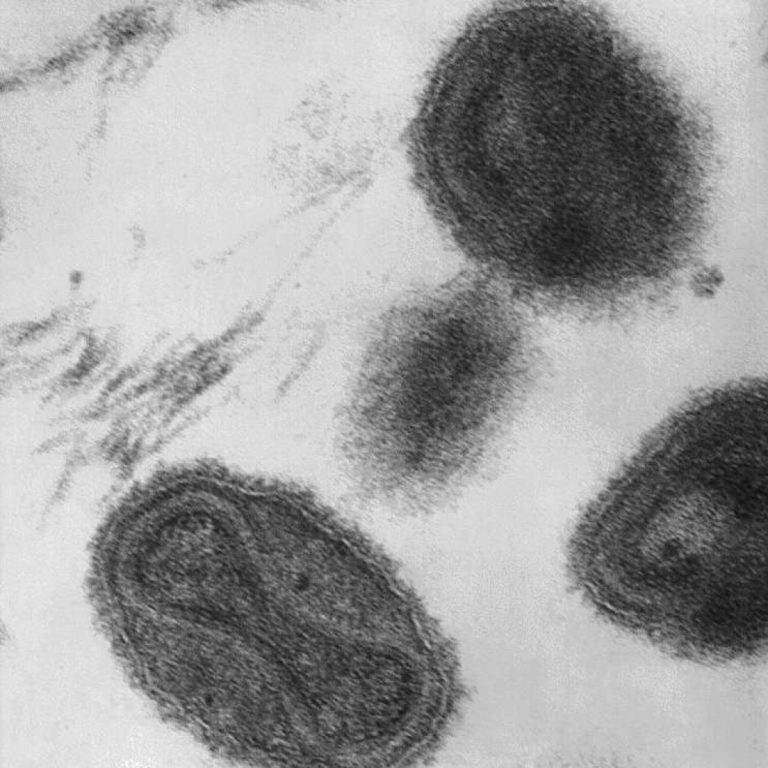
On Feb. 26, 2020, Tonix Pharmaceuticals announced a strategic collaboration with Southern Research to support the development of…
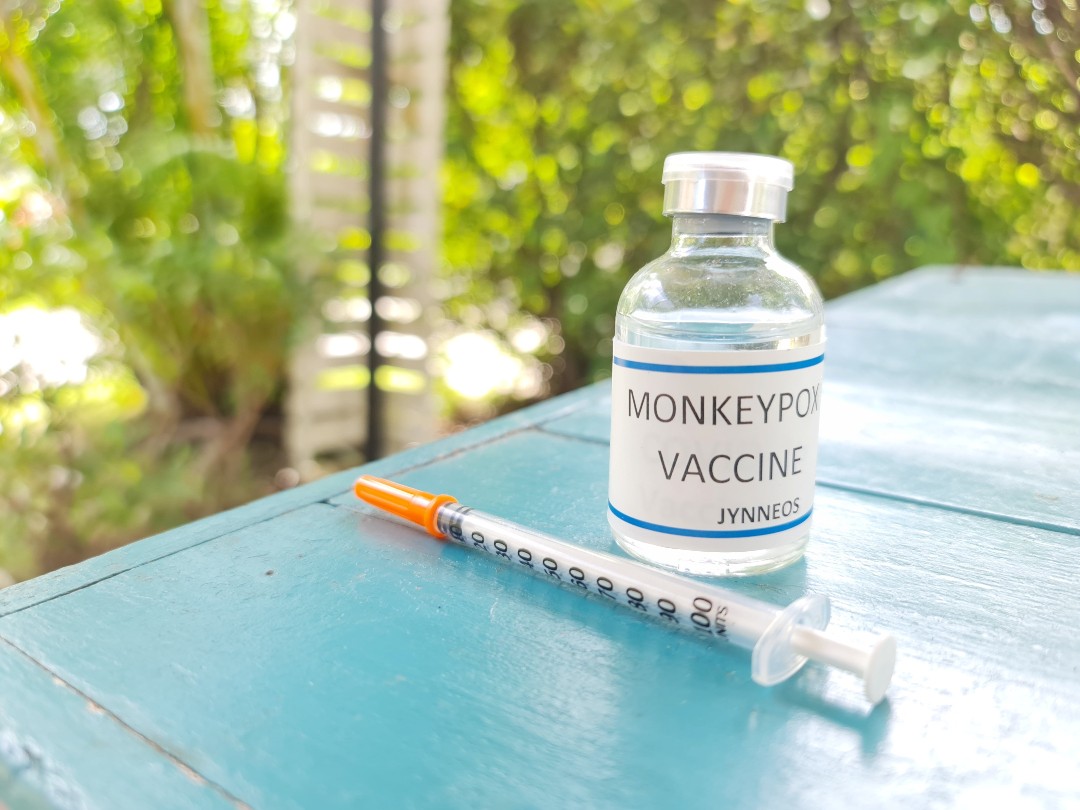
On Sept. 24, 2019, the U.S. Food and Drug Administration announced approval of Jynneos Smallpox and Monkeypox Vaccine,…

On Jul 6, 2017, a group led by virologist David Evans of the University of Alberta in Edmonton,…
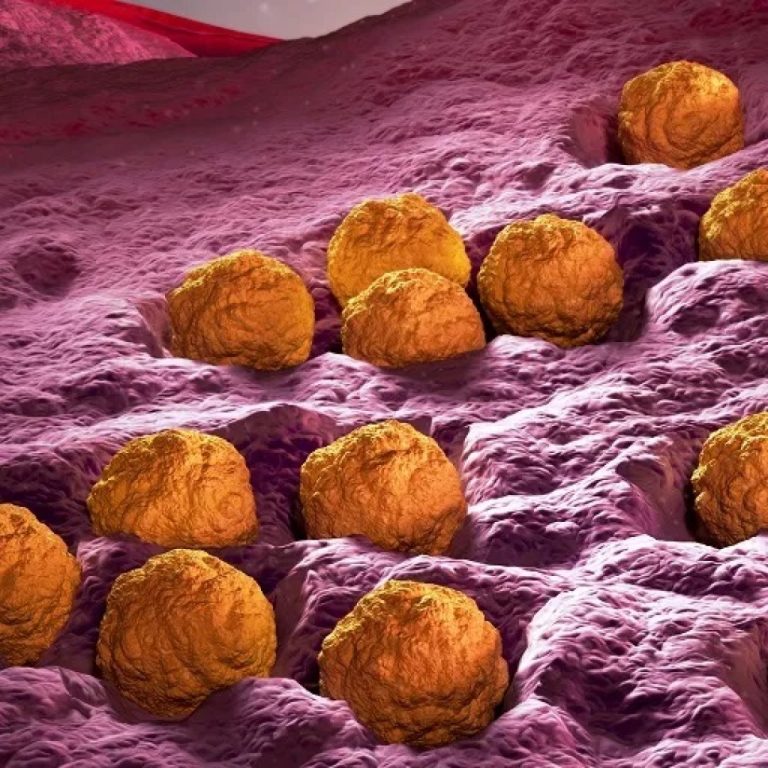
On Apr. 29, 2015, the Pan American Health Organization ᅠdeclared that the Americas region has become the first…
On Jun. 28, 2011, during the 37th United Nations Food and Agriculture Organization Conference, the 192 Member countries…
On Jul. 13, 2010, Bavarian Nordic announced that it had delivered 1 million doses of its smallpox vaccine…

On Feb. 29, 2008, the U.S. Centers for Disease Control and Prevention (CDC) announced it had begun distribution…