WHO Reports Diphtheria outbreak in African Region
On Nov. 21, 2025, the World Health Organization (WHO) reported that from 1 January to 2 November 2025,…

On Nov. 21, 2025, the World Health Organization (WHO) reported that from 1 January to 2 November 2025,…
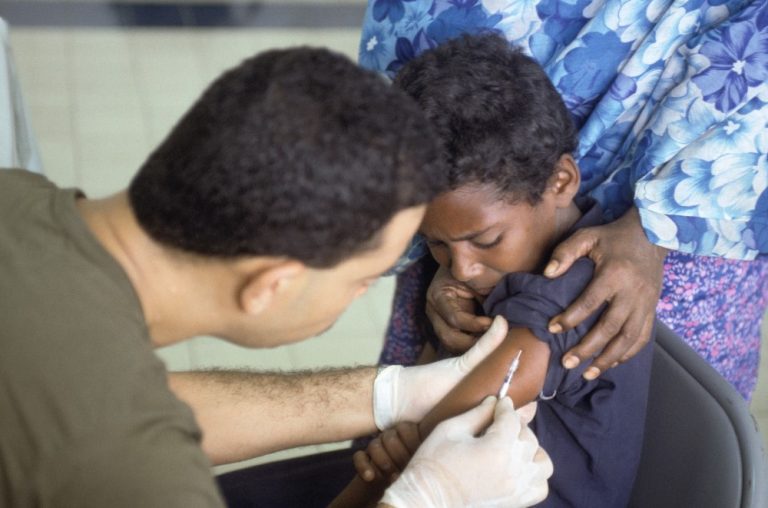
On Jul. 15, 2025, in 2024, 89% of infants globally – about 115 million – received at least…

On Jul. 9, 2025, a research team led by Eske Willerslev, professor at the University of Copenhagen and…

On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…

On Jul. 15, 2022, The largest sustained decline in childhood vaccinations in approximately 30 years has been recorded…

Around 500 BCE, Hippocrates, a pioneering physician in the history of Medicine, and considered as the principal author…

On Jun. 1, 2021, Sanofi announced that Vaxelis (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus…

On Feb. 3, 2021, Dynavax Technologies and Serum Institute of India announced that the first participant has been…

On Jun. 4, 2020, world leaders pledged an additional US$ 8.8 billion for Gavi, the Vaccine Alliance, far…

On May 22, 2020, COVID 19 disrupted life-saving immunization services around the world, putting millions of children –…

On Mar. 23, 2020, researchers at Washington University School of Medicine in St. Louis announced they were investigating…

On Jan. 14, 2019, Sanofi announced the FDA had approved the expanded use of Adacelᆴ (Tetanus Toxoid, Reduced…

On Dec. 26, 2018, Sanofi announced the U.S. Food and Drug Administration (FDA) had approved VAXELIS (Diphtheria and…

On Apr. 27, 2018, the Centers for Disease Control and Prevention (CDC) published a comprehensive summary of previously…

On Feb. 10, 2017, the Advisory Committee on Immunization Practices (ACIP) approved the Recommended Immunization Schedule for Children…

On Apr. 1, 2014, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had expanded the approved…

On Feb. 22, 2013, the Advisory Committee on Immunization Practices (ACIP) recommended that unvaccinated pregnant women receive a…

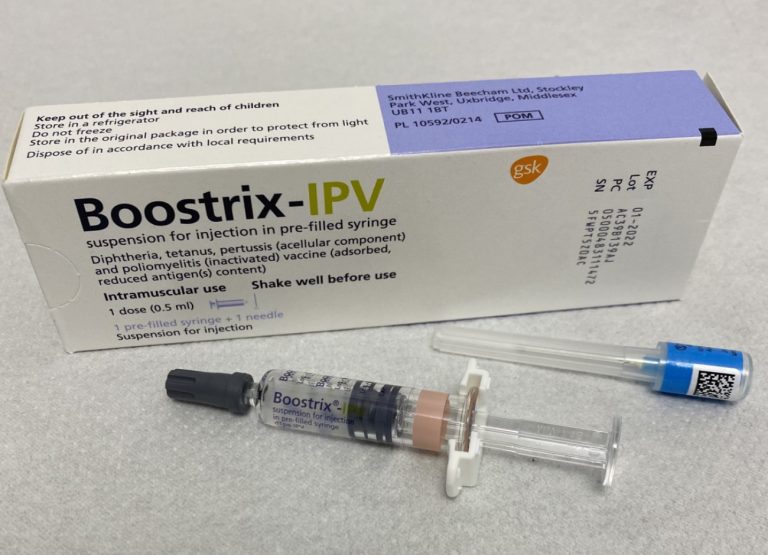
On Jul. 8, 2011, the FDA approved Boostrix (Tdap, GlaxoSmithKline) to prevent tetanus, diphtheria, and pertussis in older…
On Dec. 4, 2008, the Food and Drug Administration (FDA) approved an expanded age indication for the tetanus…

On Jun. 24, 2008, the U.S. Food and Drug Administration (FDA) licensed a combined diphtheria and tetanus toxoid…

On Jun. 23, 2008, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had licensed Pentacel, Diphtheria…

On Jun. 5, 2008, the FDA approved the use of Sanofi Pasteur’s Tenivac tetanus and diphtheria toxoids adsorbed…
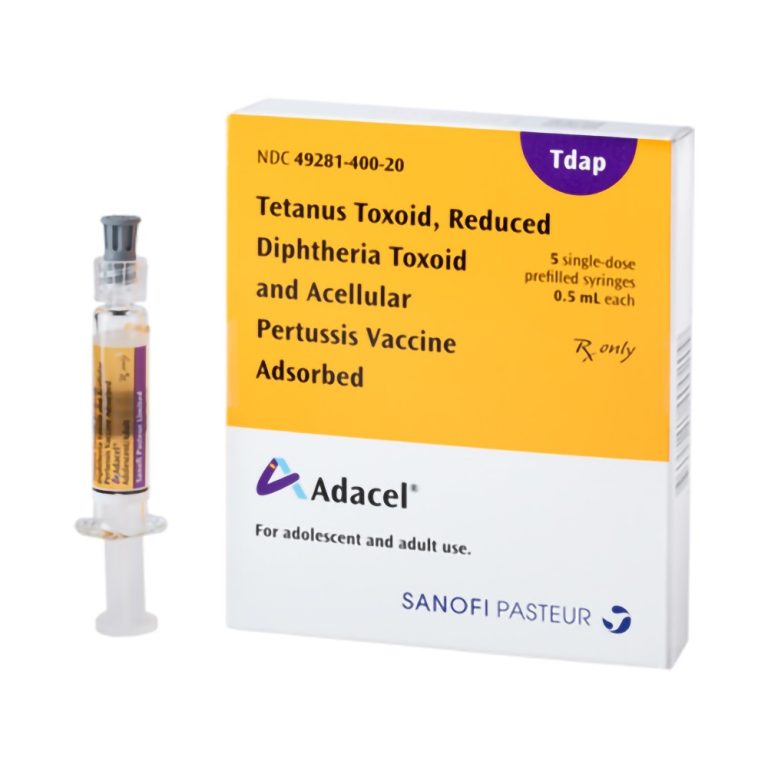
On Jun. 9, 2005, the U.S. Food and Drug Administration (FDA) licensed a 2nd Tdap vaccine (Adacel by…
On May 3, 2005, the cellular pertussis vaccine combined with the adult formulation of tetanus and diphtheria (Tdap:…
On Jan. 14, 2005, the first meningococcal polysaccharide (Serogroups A, C, Y and W-135) diphtheria toxoid conjugate vaccine…

On Mar. 24, 2004, the U.S. Food and Drug Administration (FDA) licensed tetanus and diphtheria toxoids adsorbed for…

On Dec. 13, 2002, the U.S. Food and Drug Administration (FDA) announced it had licensed a combined diphtheria…

On May 14, 2002, the Food and Drug Administration (FDA) approved for use an additional combined diphtheria and…

On Dec. 9, 1999, the diphtheria and tetanus toxoids and acellular pertussis vaccine (Tripedia by Connaught) was licensed….

On Jan. 15. 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…