World Health Organization confirmed case of human infection with avian influenza A(H5N1) virus in Australia
On May 22, 2024, the World Health Organization (WHO) announced that the first confirmed case of human infection…
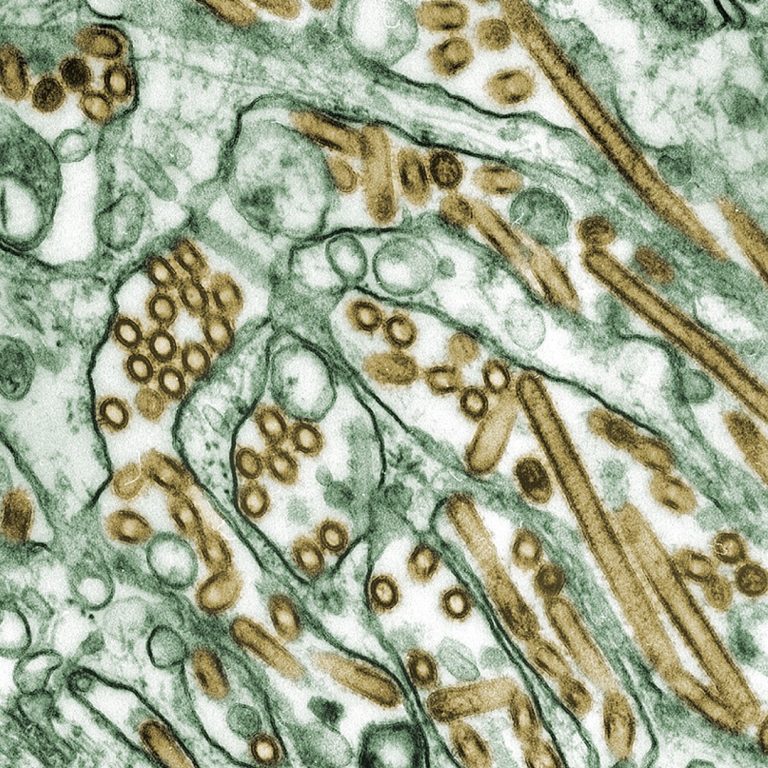
On May 22, 2024, the World Health Organization (WHO) announced that the first confirmed case of human infection…

On May 20, 2024, the Michigan Department of Agriculture and Rural Development (MDARD) announced the detection of highly…
On May 17, 2024, researchers at Washington University School of Medicine in St. Louis announced a study had…

On May 15, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…
On May 10, 2024, the National Academies of Sciences, Engineering, and Medicine reported that the COVID-19 vaccines made…
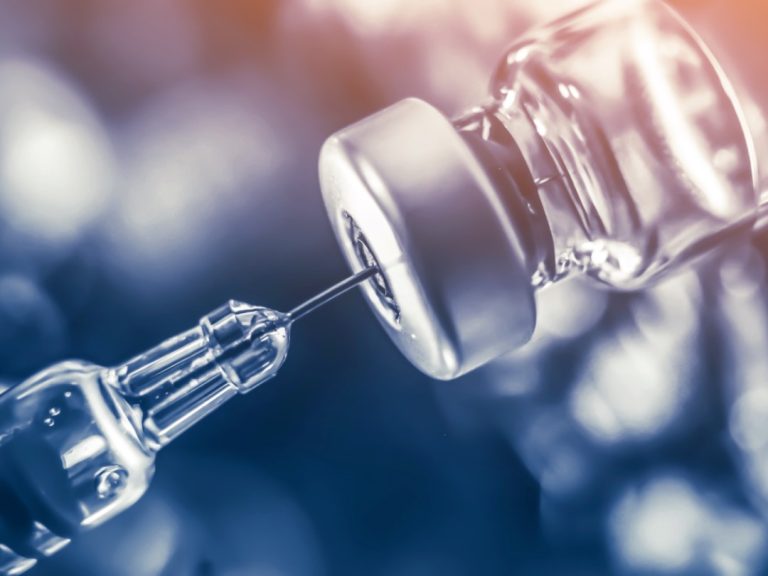
On May 10, 2024, Novavax announced that it had entered into a co-exclusive licensing agreement with Sanofi. The…
On Apr. 30, 2024, a meta-analysis of six randomized controlled trials in JAMA Neurology found no increase in seizures in…
On Apr. 23, 2024, the U.S. Food and Drug Administration (FDA) announced that nearly all (99%) of the…

On Apr. 22, 2024, Moderna announced a contract with the Ministry of Health in Brazil (Ministerio da Saude)…

On Apr. 21, 2024, United States Department of Agriculture’s (USDA) APHIS National Veterinary Services Laboratories announced it had…

On Apr. 15, 2024, scientists at University of California Riverside demonstrated a new, RNA-based vaccine strategy that is…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…
On Apr. 12, 2024, Oregon Health Sciences University (OHSU) announced research that shows using live SARS-CoV-2 virus revealed…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 1, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…
On Mar. 26, 2024, the National Academies of Sciences, Engineering, and Medicine released a report that said action…

On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…

On Mar. 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle…

On Mar. 20, 2024, the Minnesota Board of Health announced that a Stevens County goat kid (juvenile goat)…
On Mar. 14, 2024, the Institute for Health Metrics and Evaluation (IHME) released all-cause mortality, life expectancy, and…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…

On Feb. 29, 2024, Valneva announced that the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee…

On Feb. 28, 2024, the CDC Advisory Committee on Immunization Practices’ (ACIP) announced a recommendation for adults ages…

On Feb. 28, 2024, the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee on Immunization Practices…

On Feb. 22, 2024, the University of California, San Francisco (UC, San Francisco) launched the world’s first tissue…

On Feb. 21, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved $98,592,155…
On Feb. 21, 2024, the World Health Organization (WHO) reported that more than half the world’s countries will…
On Feb. 14, 2024, the National Institute of Allergy and Infectious Diseases (NIAID) reported study results that showed…