California’s Stem Cell Agency invested $44 million to advance regenerative medicine research and manufacturing in California
On Sept. 29, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $43.8 million to fund projects aimed…

On Sept. 29, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $43.8 million to fund projects aimed…

On Sep. 27, 2023, Ligand Pharmaceuticals announced that it has closed the transaction to acquire assets of Novan following…

On Sept. 26, 2023, the Advanced Research Projects Agency for Health (ARPA-H), an agency within the U.S. Department…

On Sept. 25, 2023, the Washington State Department of Health confirmed the presence of West Nile virus in…
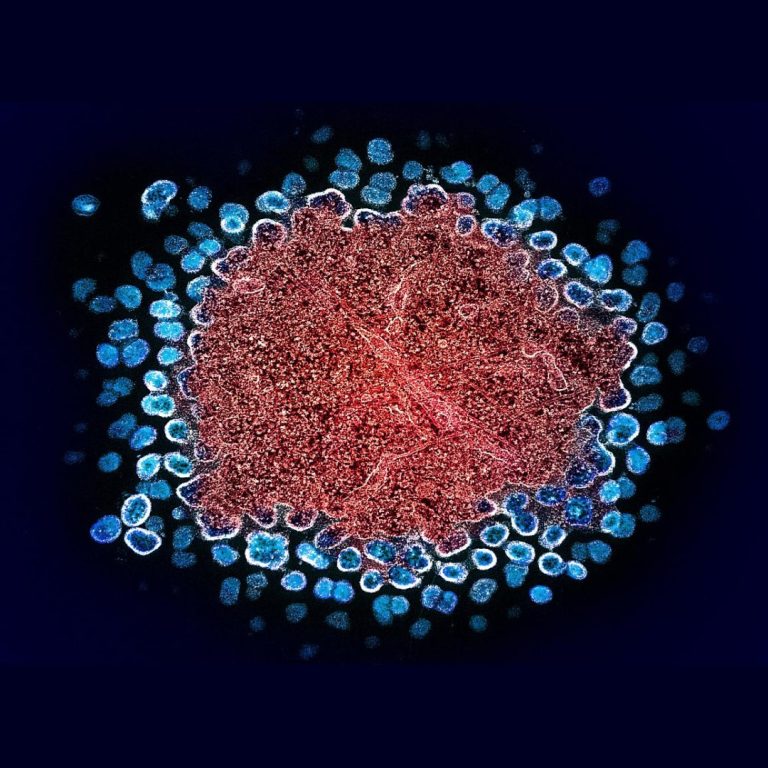
On Sept. 20, 2023, the National Institutes of Health announced that a trial of a preventive HIV vaccine…

On Sept. 14, 2023, the World Health Organization (WHO) reported that as of September 11, 2023, a total…

On Sept. 12, 2023, the National Institutes of Health announced it was establishing the Multi-Omics for Health and…

On Sept. 11, 2023, researchers at the National Institutes of Health (NIH) reported that living in an area…

On Sept. 6, 2023, the Markey Cancer Center (MCC) became Kentucky’s first and only National Cancer Institute (NCI)-Designated…

On Sept. 6, 2023, Oregon Health & Science University (OHSU) announced it had achieved a landmark in orthopaedic…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Aug. 27, 2003, The Institute for Collaborative Biotechnologies (ICB) announced it was funding a $50 million five…
On Aug. 25, 2023, Harvardメs Center for Brain Science announced the ceation of an eye atlas that pinpoints…
On Aug. 23, 2023, a gene variant found almost exclusively in the genomes of people of African ancestry…
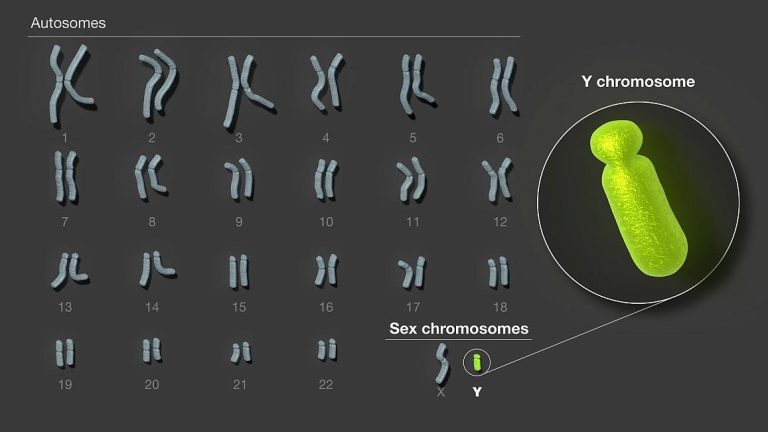
On Aug. 23. 2023, researchers co-led by University of California, Santa Cruz Assistant Professor of Biomolecular Engineering Karen…
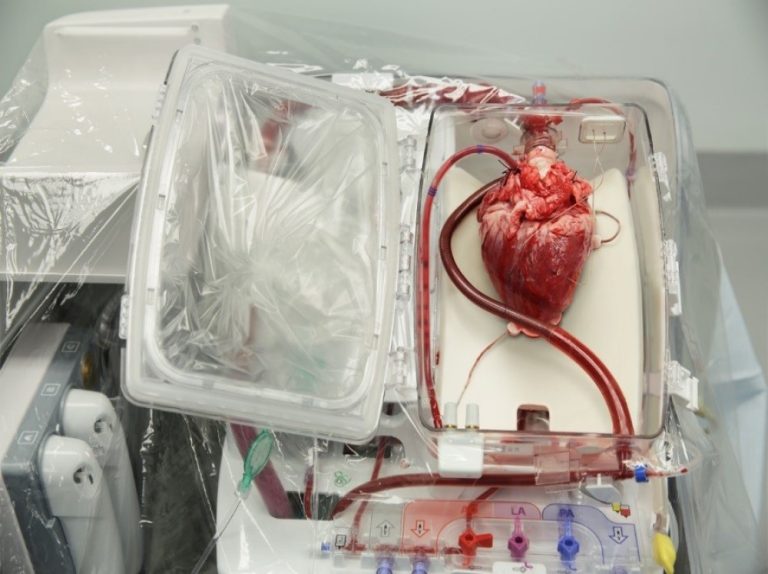
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…
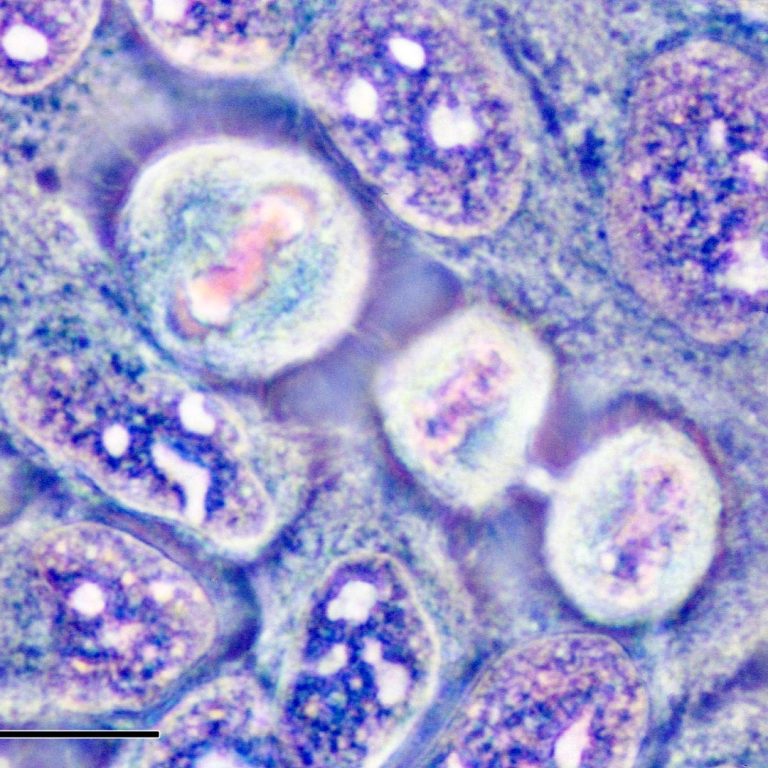
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…
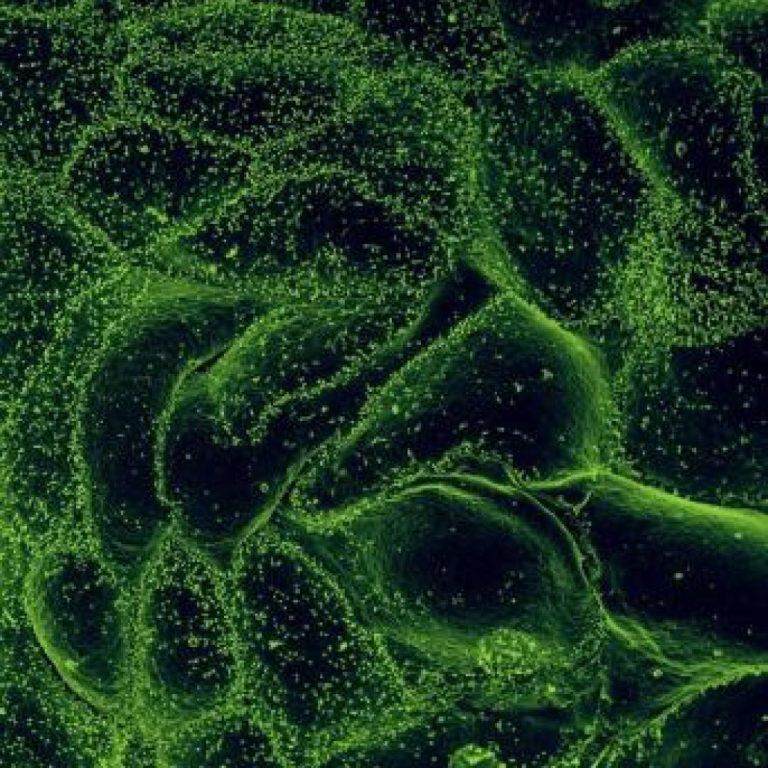
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…

On Jul. 28, 2023, in a first-of-its-kind clinical trial, bioelectronic medicine researchers, engineers and surgeons at Northwell Healthメs…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…

On Jul. 27, 2023, SIGA Technologies announced that the U.S. Department of Health and Human Services exercised procurement…
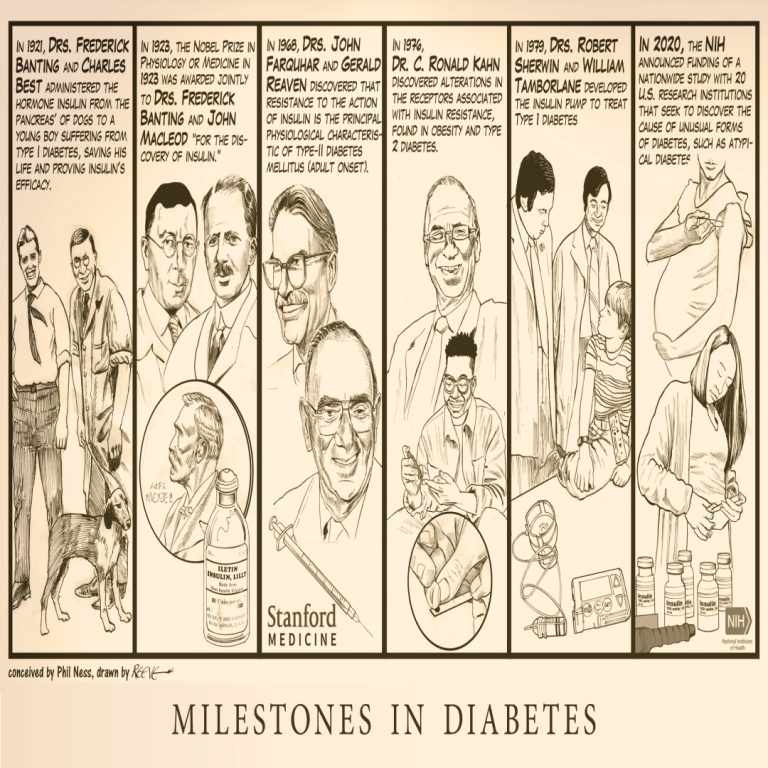
Milestones in Diabetes illustrates major milestones from the first administration of the hormone insulin in 1921 to a…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…

On Jul. 19, 2023, the National Institute of Health announced that a nationwide research team had created the…
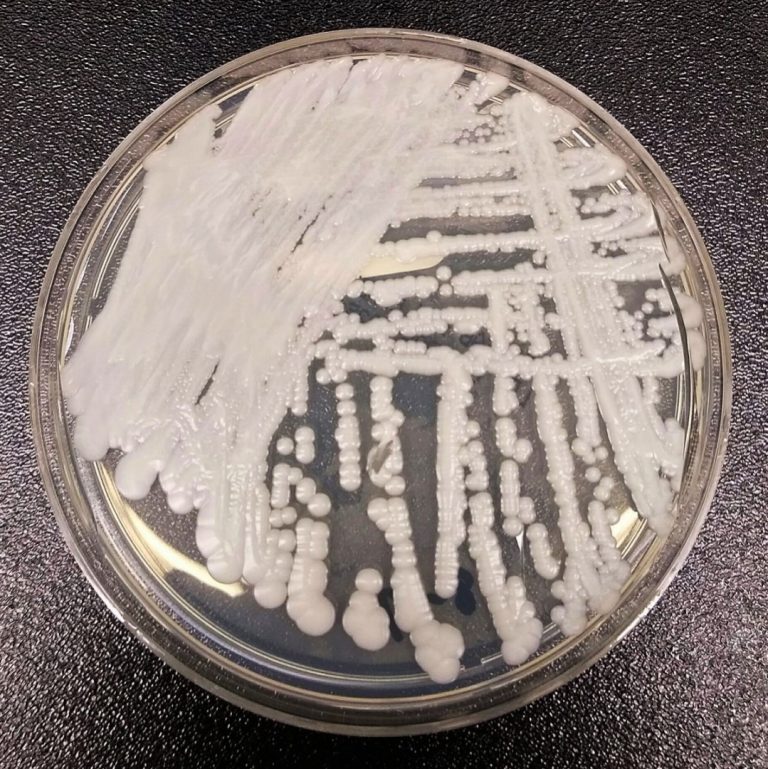
On Jul. 19, 2023, the King County Department of Health issued a Health Advisory that Candida auris (C….

On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 14, 2023, the UK Animal and Plant Health Agency reported that additional asymptomatic human detections of…