FDA declined to issue Rigel Pharma EUA for fostamatinib for teatment of COVID-19 in hospitalized adults
On Aug. 13, 2021, Rigel Pharma announced that the U.S. Food and Drug Administration (FDA) had informed the…

On Aug. 13, 2021, Rigel Pharma announced that the U.S. Food and Drug Administration (FDA) had informed the…
On Aug. 11, 2021, the National Institutes of Health (NIH) announced that one dose of a new monoclonal…
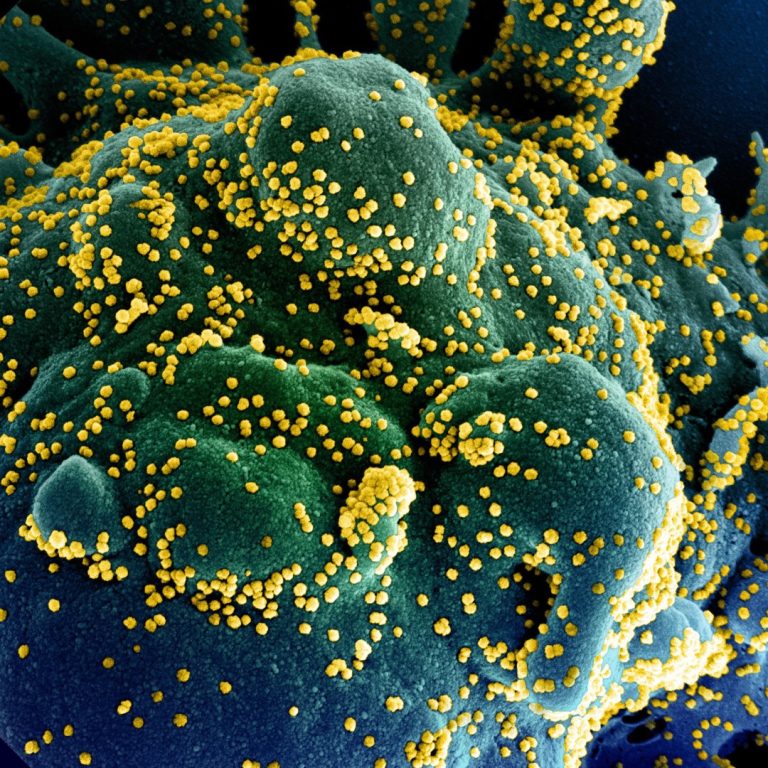
On Aug. 10, 2021, the National Institutes of Health (NIH) announced that a pilot study had begun to…

On Jul. 30, 2021, Humanigen announced that the NIH had advanced the ACTIV-5/BET-B study to a Phase 2/3…

On Jul. 27, 2021, Oragenics announced it had entered into a licensing agreement with the National Research Council…

On Jul. 26, 2021, Trevena announced that the first COVID-19 patient had been enrolled in the NIH-funded ACTIV-4…
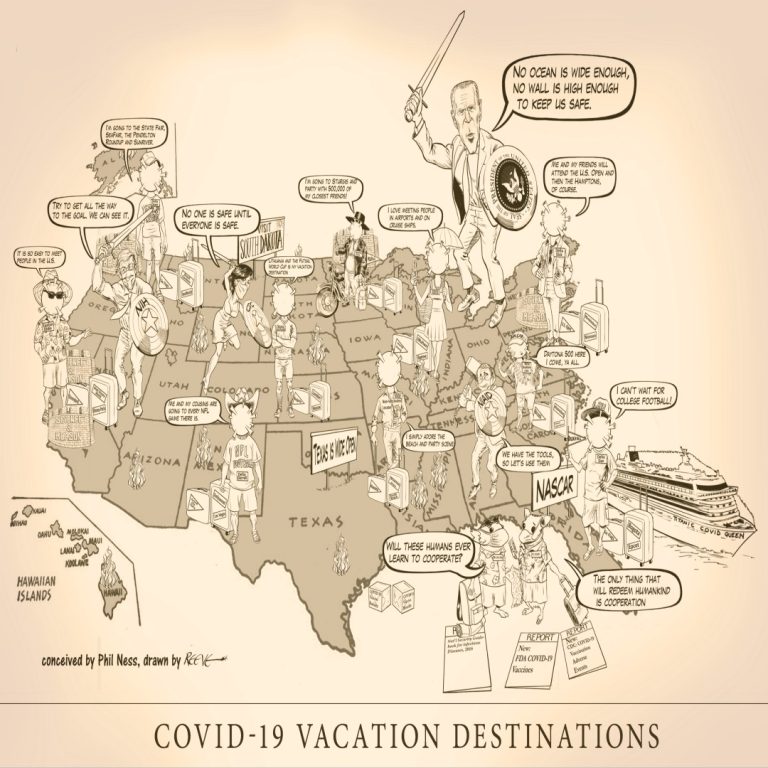
Planning a Summer vacation? Where to go? How about going to one of those large events with tens…

On Jul. 22, 2021, the National Institutes of Health announced it had renewed grants to seven regional centers…

On Jul. 8, 2021, the National Institutes of Health (NIH) announced that a new study authored by scientists…
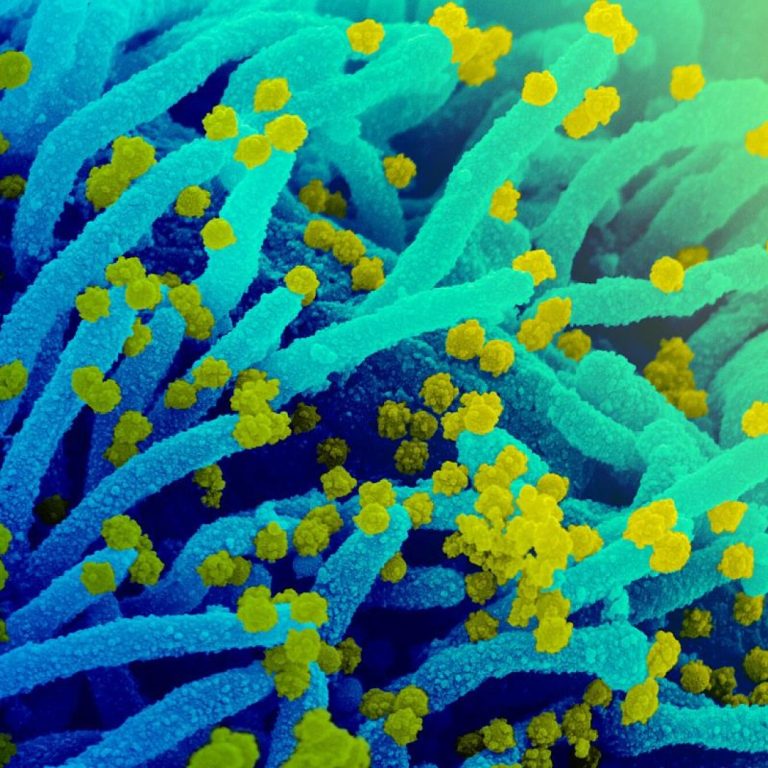
On Jun. 30, 2021, the National Institutes of Health announced a highly anticipated study that compared rapid antigen…

On Jun. 29, 2021, Rigel Pharma announced that fostamatinib, the Company’s novel oral spleen tyrosine kinase (SYK) inhibitor,…

On Jun. 17, 2021, the Biden Administration is investing more than $3 billion to accelerate the discovery, development…

On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…

On Jun. 3, 2021, the National Institutes of Health announced that the experimental drug TEMPOL may be a…

On Jun. 1, 2021, the U.S. Department of Health and Human Services (HHS) unveiled a new type of…
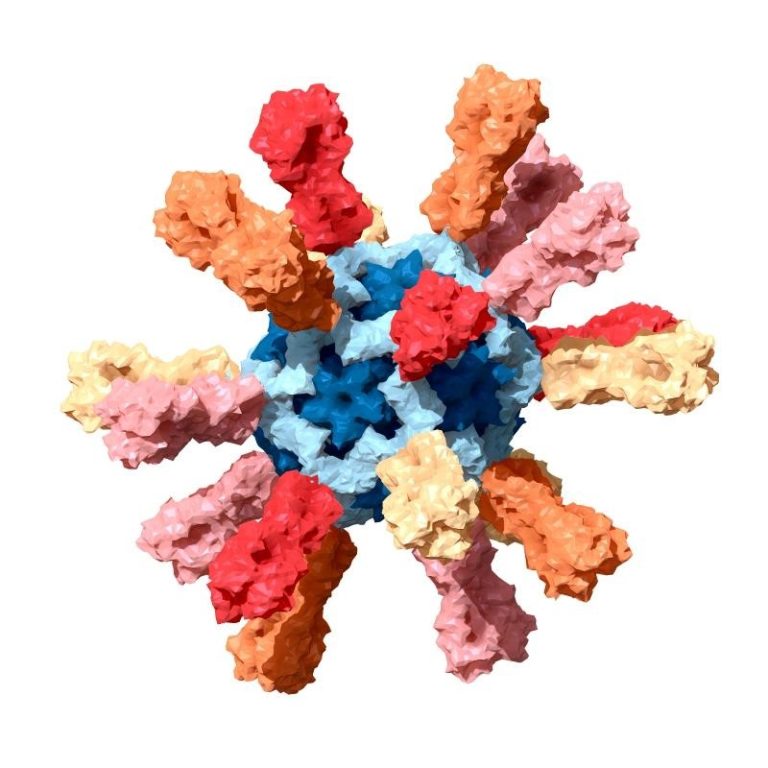
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…
On May 26, 2021, National Institutes of Health (NIH) scientists reported that the immune system’s attempt to eliminate…

On May 6, 2021, Trevena announced that TRV027, the Companyメs novel AT1 receptor selective agonist, had been selected…

On Apr. 29, 2021, the National Institutes of Health announced it was funding $29 million in additional grants…

COVID-19 and it’s naysayers are attacking Science and Reason. The defenders must suffer the slings and arrows from…

On Apr. 23, 2021, the NIH announced that a study assessing how people with immune system deficiencies or…
On Apr. 22, 2021, the NIH announced that a phase 3 trial to test the safety and efficacy…

On Apr. 21, 2021, the NIH announced that a Phase 2/3 trial to evaluate a new fully-human polyclonal…

On Apr. 19, 2021, the NIH announced that it was funding a large, randomized, placebo-controlled Phase 3 clinical…

On Apr. 15, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had had established…
On Apr. 14, 2021, National Institutes of Health researchers reported that a study which compared to newborns conceived…

On Apr. 13, 2021, Rigel Pharma announced the completion of patient enrollment in a multi-center Phase 2 clinical…

On Apr. 9, 2021, Regeneron announced that newly updated National Institutes of Health (NIH) COVID-19 treatment guidelines strongly…

On Apr. 7, 2021, the National Institutes of Health (NIH) announced that a clinical trial was underway to…

On Apr. 7, 2021, Moderna announced publication of antibody persistence data out to 6 months following the second…