Texas Life Science Genealogy, 2018
Texas Life Science Genealogy illustrates life science companies and non-profit research organization in the State of Texas by…
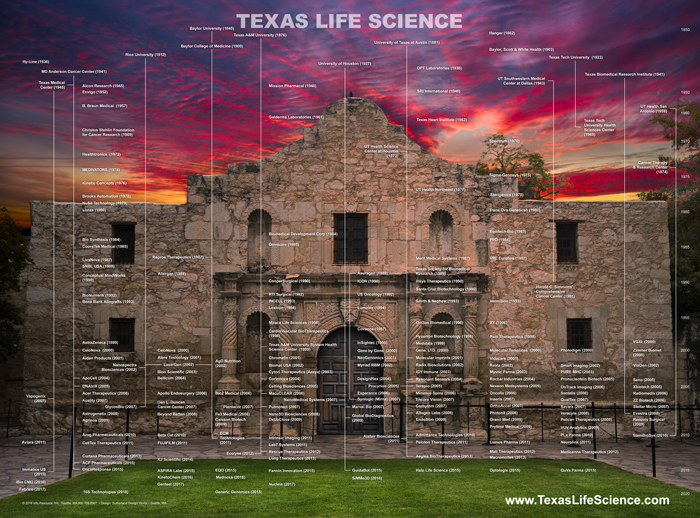
Texas Life Science Genealogy illustrates life science companies and non-profit research organization in the State of Texas by…

Oregon BioEvolution illustrates life science companies and non-profit research organization in the State of Oregon by the year…

On May 24, 2018, the United States Food and Drug Administration (U.S. FDA) released a statement on GR2E…

On May 16, 2018, the Biocomplexity Institute’s long-anticipated new data center, built with an internal investment of $5…
On May 14, 2018, University of California, Los Angeles (UCLA) researchers characterized the mechanism of the mammalian EAK-7…

On Apr. 27, 2018, the Centers for Disease Control and Prevention (CDC) published a comprehensive summary of previously…

Alberta Life Science Genealogy illustrates life science companies and non-profit research organizations in the Province of Alberta by…

On Apr. 10, 2018, Amgen announced plans to build a new state-of-the-art next-generation biomanufacturing plant at its 75-acre…

On Feb. 28, 2018, The MMK Foundation, founded by Mark and Marcia King, pledged $1 million to be…
On Feb. 9, 2018, the U.S. Centers for Disease Control and Prevention (CDC) published the 2018 U.S. recommended…

On Feb. 9, 2018, the U.S. Centers for Disease Control and Prevention (CDC) published the 2018 U.S. recommended…

On Jan. 24, 2018, Chinese scientists announced they had produced two genetically identical long-tailed macaques using the same…

In 2018, Rallybio was founded as a privately-held, development-stage biotechnology company focused on bringing life-transforming medicines to patients…

On Dec. 5, 2017, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) had approved Ozempic…
On Nov. 28, 2017, surgeons at University of Washinton Medicine in Seattle announced they had performed the first…

On. Nov. 13, 2017, research by Dr. Patrick McGovern, Director of the Biomolecular Archaeology Laboratory at the University…

On Nov. 10, 2017, the Wyss Medical Foundation pledged $2 million to support James P. Stannard as the…

On Nov. 9, 2017, Dynavax Technologies announced that the U.S. Food and Drug Administration (FDA) had approved HEPLISAV-B…

On Nov. 9, 2017, UNMC announced that Jingwei Xie, Ph.D, a biomedical engineer, had been awarded a four-year,…

On Dec. 1, 2017, Quest Diagnostics announced it acquired Cleveland HeartLab, a leader in innovative diagnostic services for…

On Nov. 1, 2017, a study from the Earth Microbiome Project reported the most extensive snapshot ever of…
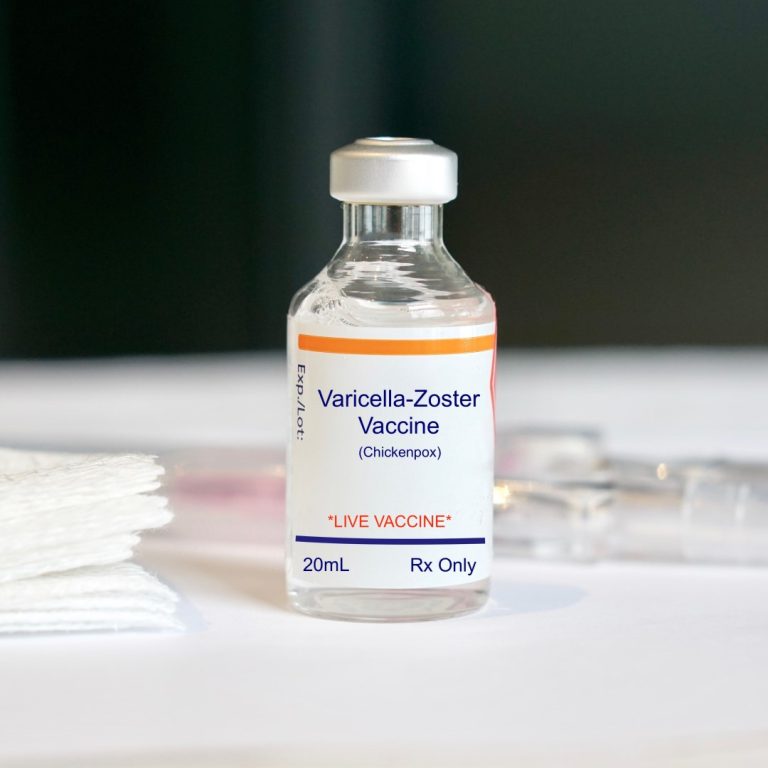
On Oct. 23, 2017, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Shingrix (Zoster Vaccine…

On Oct. 17, 2017, Norman “Ned” Sharpless was sworn in as the 15th Director of the National Cancer…

On Oct. 12, 2017, the NIH and 11 leading biopharmaceutical companies launched the Partnership for Accelerating Cancer Therapies…

On Oct. 11, 2017, researchers funded by the National Institutes of Health (NIH) announced they had completed a…

On Oct. 2, 2017, The Nobel Assembly announced that Michael Rosbash, a HHMI investigator, Jeffrey Hall of Brandeis…

On Aug. 30, 2017, the U.S. Food and Drug Administration (FDA) approved Kymriah (tisagenlecleucel) for certain pediatric and…

On Jul. 8, 2017, Chinaメs State Council released its モNew Generation AI Development Planヤ to advance Chinese artificial…

On Jun. 13, 2017, the National Institutes of Health (NIH) awarded a $6 million grant to Cleveland Clinic…

On Jun. 7, 2017, the W. M. Keck Foundation gave $10 million to support a USC Health Sciences…