iBio reported successful preclinical immunization studies with next-gen nucleocapsid COVID-19 vaccine
On Jul. 15, 2021, iBio announced that preclinical studies of IBIO-202, its subunit vaccine candidate that targets the…

On Jul. 15, 2021, iBio announced that preclinical studies of IBIO-202, its subunit vaccine candidate that targets the…

On Jul. 14, 2021, Hologic announced that it had obtained a CE Mark for the use of saliva…

On Jul. 13, 2021, the Food and Drug Administration (FDA) revisions to the vaccine recipient and vaccination provider…

On Jul. 13, 2021, RELIEF THERAPEUTICS reported that its collaboration partner, NRx Pharmaceuticals, and Quantum Leap Healthcare Collaborativeル…

On Jul. 12, 2021, Gavi, the Vaccine Alliance, announced that it had signed advance purchase agreements with Sinopharm…

On Jul. 12, 2021, BioNTech announced that Fosun Industrial Co. had reached advance procurement agreements for the mRNA-based…

On Jul. 9, 2021, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to Ortho-Clinical…

On Jul. 9, 2021, Humanigen announced its submission for Marketing Authorization for lenzilumab in COVID-19, begun in Jun….

On Jul. 9, 2021, a federal jury convicted a timber thief who authorities said started a large forest…

On Jul. 8, 2021, the National Institutes of Health (NIH) announced that a new study authored by scientists…

On Jul. 8, 2021, Pfizer and BioNTech announced encouraging data in the ongoing booster trial of a third…

On Jul. 7, 2021, Rice University announced a study to develop an inhalable COVID-19 vaccine. The project led…
On Jul. 6, 2021, the World Health Organization announced updated patient care guidelines to include interleukin-6 receptor blockers,…

On Jul. 6, 2021, Dynavax Technologies and Biological E announced the execution of a commercial supply agreement of…

On Jul. 5, 2021, CerTest Biotec and BD announces addition of saliva to the CE Marked VIASURE SARS-CoV-2…

On Jul. 2, 2021, the National Institutes of Health funding five additional projects to identify ways of safely…

On Jul. 2, 2021, the Food and Drug Administration (FDA) authorized the use, under the emergency use authorization…

On Jul. 1, 2021, HDT Bio announced the U.S. Food and Drug Administration had reviewed the companyメs Investigational…

On Jun. 30, 2021, Atea Pharmaceuticals announced positive interim results from the global Phase 2 study evaluating AT-527…
On Jun. 30, 2021, CureVac announced results from the final analysis of its 40,000 subject international pivotal Phase…
On Jun. 30, 2021, the National Institutes of Health announced that two U.S. Phase 1 clinical trials of…

On Jun. 30, 2021, XPhyto announced that it had signed a master supply agreement with Beovita and Tackleberries,…

On Jun. 30, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Clover…

On Jun. 29, 2021, Rigel Pharma announced that fostamatinib, the Company’s novel oral spleen tyrosine kinase (SYK) inhibitor,…

On Jun. 29, 2021, the National Institutes of Health announced that an adjuvant developed with its funding had…

On Jun. 29, 2021, Altimmune announced phase 1 AdCOVID clinical trial data that showed AdCOVID appeared to be…

On Jun. 29, 2021, VBI Vaccines announced positive Phase 1 data from its Phase 1/2 trial of the…

On Jun. 28, 2021, the University of Oxford in partnership with AstraZeneca began vaccinations for a new phase…

On Jun. 28, 2021, Meridian Bioscience announced that it had re-submitted its application for Emergency Use Authorization (EUA)…
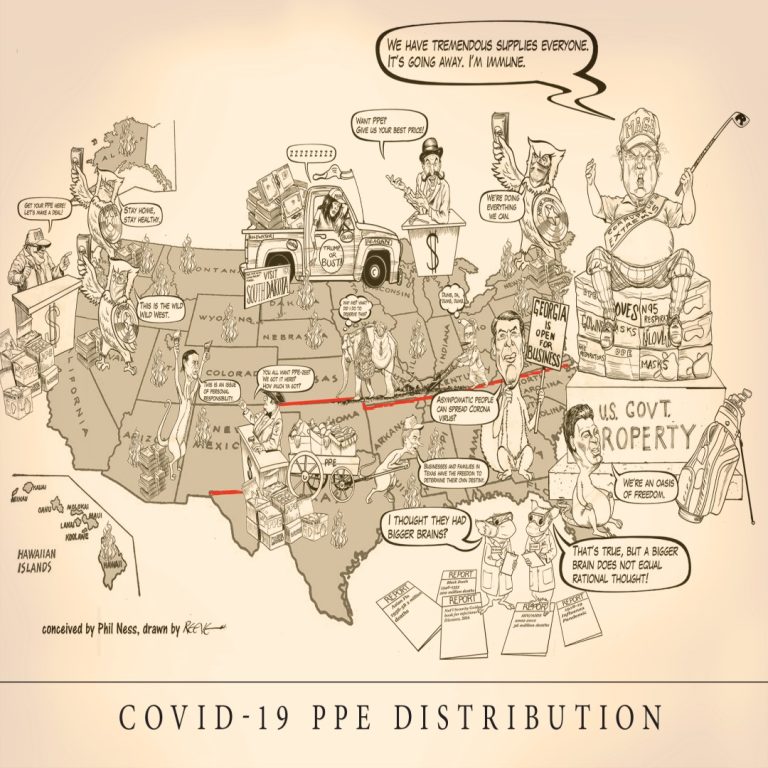
Cast of Characters: Jay Inslee, Governor, Washington | Gavin Newsom, Governor, California | Doug Ducey, Governor, Arizona |…