Pfizer announced positive top-line data of phase 3 global trial for bivalent RSV vaccine candidate
On Nov. 1, 2022, Pfizer announced positive top-line data from the Phase 3 clinical trial (NCT04424316) MATISSE (MATernal…

On Nov. 1, 2022, Pfizer announced positive top-line data from the Phase 3 clinical trial (NCT04424316) MATISSE (MATernal…
On Nov. 1, 2022, the Medicines Control Authority of Zimbabwe announced that it had approved the use of…

On Nov. 1, 2022, study results published in JAMA found that estimates in this cross-sectional study of 694,660…

On Oct. 31, 2022, the National Institutes of Health announced that a clinical trial had found that one…

On Sept. 3, 2014, the Galien Foundation, the premier global institution dedicated to honoring innovators in life sciences,…
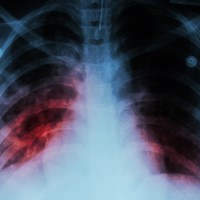
On Oct. 27, 2022, the World Health Organization reported that an estimated 10.6 million people fell ill with…

On Oct. 26, 2022, the NIH announced it had awarded the University of Southern California (USC) $11.7 million…

On Oct. 25, 2022, the University of Washington reported that daily global COVID-19 infections were projected to rise…

On Oct. 25, 2022, the National Institutes of Health (NIH) announced that the University of Missouri had been…

On Oct. 24, 2022, Merck announced that the European Commission had approved an expanded indication for VAXNEUVANCEル (Pneumococcal…
On Oct. 20, 2022, newly published results supported previous findings that the recombinant zoster vaccine Shingrix, recommended by…
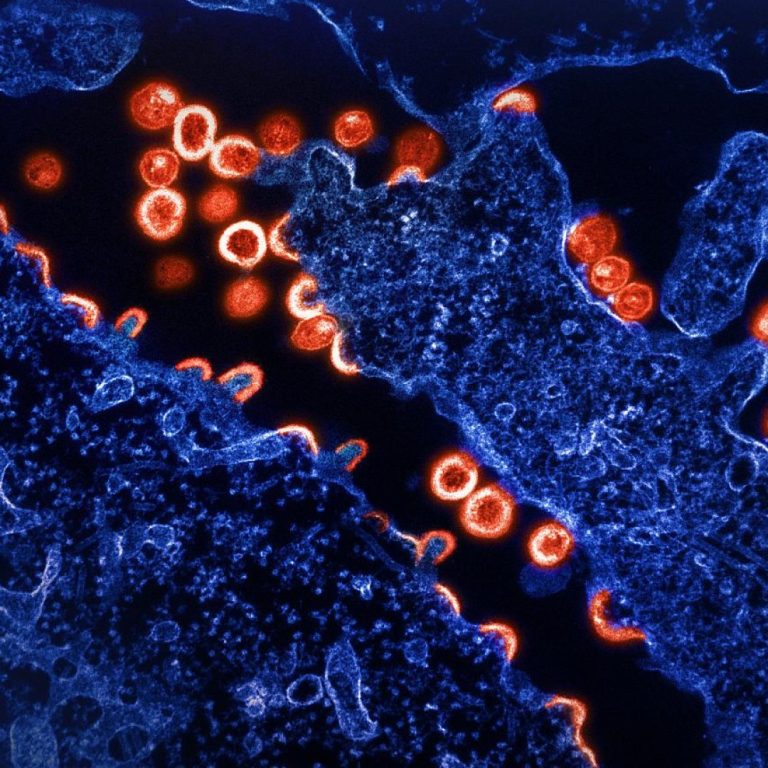
On Oct. 19, 2022, Pfizer and BioNTech announced that the European Medicines Agencyメs (EMA) Committee for Medicinal Products…
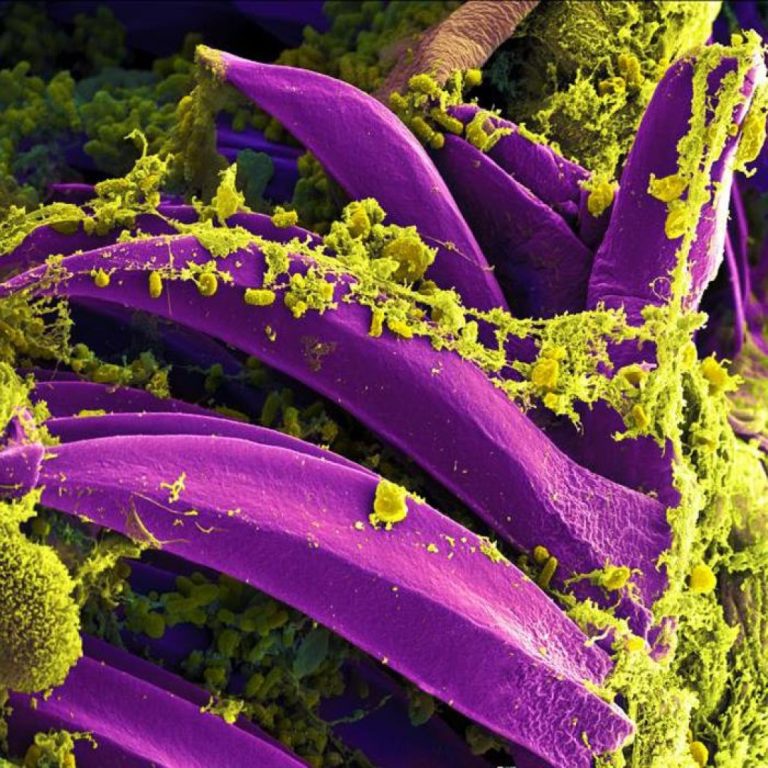
On Oct. 15, 2022, scientists reported that DNA evidence showed that the plague infection was endemic in the…

On Oct. 13, 2022, researchers from the University of Oxford reported findings from a study exploring how certain…

On Oct. 12, 2022, scientists announced they had developed a research method that allows for a much more…

On Oct. 11, 2022, researchers from the University of Oxford have reported new findings from a Phase 1…

On Oct. 11, 2022, Roche announced the launch of its next-generation portfolio SARS-CoV-2 rapid antigen tests (モ2.0ヤ) for…

On Oct. 7, 2022, the U.S. Food and Drug Administration approved Boostrix (Tetanus Toxoid, Reduced Diphtheria Toxoid and…
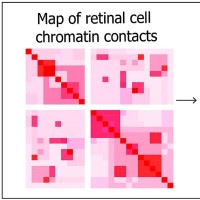
On Oct. 7, 2022, the National Eye Institute researchers announced they had mapped the organization of human retinal…

On Oct. 6, 2022, the World Health Organization announced the first cholera outbreak in nearly three decades in…

On Oct. 6, 2022, Merck announced data from two real-world evidence studies evaluating LAGEVRIOル (molnupiravir), an investigational oral…

On Oct. 5, 2022, researchers at UC Berkeley reported that coccidioidomycosisラalso known as Valley feverラan infectious disease, was…

On Oct. 5, 2022, the Nobel Prize in Chemistry was awarded jointly to Carolyn R. Bertozzi, Morten Meldal…
On Oct. 4, 2022, Washington University in St. Louis announced it had licensed the rights to develop, manufacture…

On Oct. 3, 2022, Monica M. Bertagnolli, M.D., started as the 16th director of the National Cancer Institute….

On Sept. 30, 2022, the U.S. Food and Drug Administration Modernization Act reauthorized the Prescription Drug User Fee…
On Sept. 28, 2022, Pfizer and BioNTech announced they had completed a submission to the European Medicines Agency…

On Sept. 23, 2022, researchers at Tufts University announced they had developed a method to make silk-based materials…

On Supt. 22, 2022, with a five-year, $126 million grant from the National Institutes of Health (NIH), a…

On Sept. 21, 2022, the AACR released the 12th edition of its AACR Cancer Progress Report, an annual…