Two fatal human influenza A/H5N1 (Bird Flu) virus infections reported in Cambodia
On Oct. 12, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that two fatal human…

On Oct. 12, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that two fatal human…

On Oct. 11, 2023, the European Commission, the European Investment Bank and the Bill & Melinda Gates Foundation…

On Oct. 3, 2023, Vir Biotechnology announced that the Biomedical Advanced Research and Development Authority (BARDA), part of…

On Oct. 3, 2023, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of…

On Oct. 3, 2023, Novavax announced that it’s COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601) had received Emergency Use…

On Oct. 2, 2023, France began vaccinating ducks against bird flu to try and stem the virus that…

On Sept. 29, 2023, The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced it…

On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 28, 2023, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) announced…

On Sept. 27, 2023, Gritstone bio announced that it was awarded a contract by the Biomedical Advanced Research…

On Sept. 25, 2023, the Washington State Department of Health confirmed the presence of West Nile virus in…

On Sept. 15, 2023, researchers at the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…
![European Commission expanded Merck’s ERVEBO [Ebola Zaire vaccine] indication to include children 1 year of age and older](https://lifesciencehistory.com/wp-content/uploads/2022/08/european_commission_logo-768x768.jpg)
On Sept. 7, 2023, Merck announced that the European Commission (EC) had approved an expanded indication for ERVEBO…

On Sept. 1, 2023, the Washington State Department of Health announced an outbreak of avian influenza, also known…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Aug. 27, 2003, The Institute for Collaborative Biotechnologies (ICB) announced it was funding a $50 million five…

On Aug. 25. 2023, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…

On Aug. 1, 2023, the Finnish Food Authority specified the euthanasia criteria for fur animals infected with avian…

On Aug. 1, 2023, BD announced that the U.S. Food and Drug Administration (FDA) 510(k) had provided clearance…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
On Jul. 28, 2023, the World Health Organization (WHO) reported circulating vaccine-derived poliovirus type 2 (cVDPV2) in the…

On Jul. 27, 2023, the IHR National Focal Point of Poland notified WHO of unusual deaths in cats…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…
On Jul. 19, 2023, the King County Department of Health issued a Health Advisory that Candida auris (C….
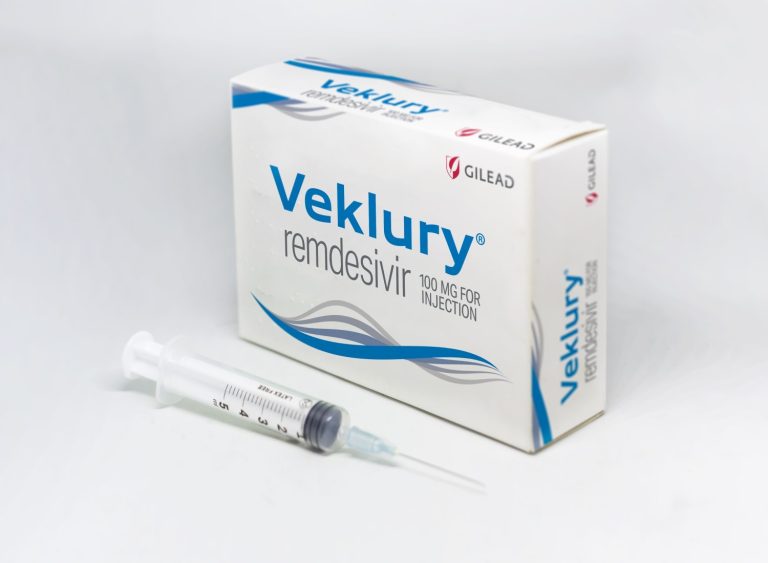
On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 14, 2023, the UK Animal and Plant Health Agency reported that additional asymptomatic human detections of…