The FDA reported false positive results with BD SARS-CoV-2 reagents for the BD Max System
On Jul. 7, 2020, the FDA alerted clinical laboratory staff and health care providers of an increased risk…

On Jul. 7, 2020, the FDA alerted clinical laboratory staff and health care providers of an increased risk…

On Jul. 7, 2020, LabCorpᆴ launched the LabCorp At Home COVID-19 Test Collection Service, the first seamless digital…

On Jul. 7, 2020, Corvus Pharmaceuticals announced that it has initiated a Phase 1 study to investigate a…

On Jul. 6, 2020, ADMA Biologics announced the commencement of operations and initiation of collections at its newest…
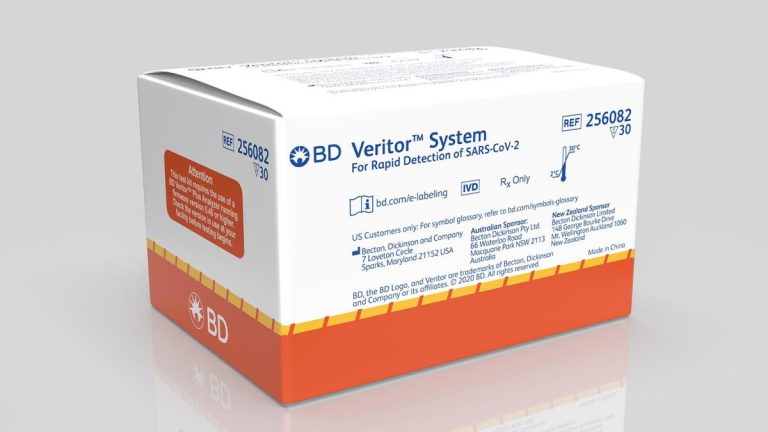
On Jul. 6, 2020, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) had granted Emergency…

On Jul. 6, 2020, the FDA issued an emergency use authorization (EUA) for the third diagnostic test for…

On Jul. 6, 2020, Endo International announced that it received FDA approval of Qwo (collagenase clostridium histolyticum-aaes) for…

On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) approved Rukobia (fostemsavir), a new type of…

On Jul. 2, 2020, Royal Philips announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Jul. 2, 2020, ViiV Healthcare, a GSK company, with Pfizer and Shionogi Limited, announced the FDA had…

On Jul. 1, 2020, MediciNova announced that the Investigational New Drug Application (IND) for MN-166 (ibudilast) for prevention…

On Jul. 1, 2020, T2 Biosystems announced completion of validation of its COVID-19 molecular diagnostic test, the T2SARS-CoV-2…

On Jul. 1, 2020, InBios announced that it had received Emergency Use Authorization (EUA) from the FDA for…

On Jun. 30, 2020, AquaBounty Technologies announced it had successfully commenced the commercial-scale harvest of conventional Atlantic salmon…

On Jun. 29, 2020, Luminex announced that the company had submitted an Emergency Use Authorization request to the…

On Jun. 25, 2020, the U.S. Food and Drug Administration (FDA) approved Fintepla (fenfluramine), a Schedule IV controlled…

On Jun. 23, 2020, the FDA launched Project Patient Voice, an initiative of the FDAメs Oncology Center of…

On Jun. 23, 2020, Takeda Pharmaceutical announced that the FDA had approved the company’s submission for its biologics…

On Jun. 18, 2020, the FDA announced participation in the COVID-19 Diagnostics Evidence Accelerator, a multi-stakeholder collaborative project…

On Jun. 18, 2020, the U.S. Food and Drug Administration (FDA) approved Ultragenyx Pharmaceutical ‘s Crysvita (burosumab-twza) injection…

On Jun. 17, 2020, Aclaris Therapeutics announced that the FDA had allowed an investigational new drug application to…

On Jun. 16, 2020, the FDA revoked the emergency use authorization (EUA) of the Chembio Diagnostic System DPP…
On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) warned health care providers about a newly…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) revoked the emergency use authorization (EUA) that…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) permitted marketing of the first game-based digital…

On Jun. 15, 2020, SIGA Technologies announced the U.S. Department of Defense (DoD) increased research and development funding…

On Jun. 12, 2020, ViiV Healthcare, a GSK company, with Pfizer and Shionogi Limited, announced the FDA had…

On Jun. 12, 2020, the FDA announced it had approved an expanded indication for Merck’s GARDASIL9 for the…

On Jun. 11, 2020, InBios announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…