Novavax 2024-2025 COVID-19 Vaccine Available at Major U.S. Pharmacies
On Sep. 13. 2024, Novavax announced that doses of the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) to…

On Sep. 13. 2024, Novavax announced that doses of the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) to…

On Sept. 13, 2024, researchers from the Wellcome Sanger Institute announced they had sequenced the genome of the…

On Sept. 13, 2024, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Ocrevus Zunovo™…

On Sept. 13, 2024, CSL Seqirus and sa-mRNA pioneer Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor…

On Sept. 12, 2024, the Finnish Institute for Health and Welfare (THL) reported that Poliovirus had been found…

On Sep. 11, 2024, the Translational Genomics Research Institute (TGen), part of City of Hope, announced the launch…

On Sept. 11, 2024, an international team of scientists led by the Leibniz Institute on Aging announced they…

On Sep. 11, 2024, researchers at UTHealth Houston and Baylor College of Medicine reported that Avian influenza A(H5N1)…
On Sep. 10, 2024, researchers at Weill Cornell Medicine in Qatar announced they had created a free-to-access online…

On Sept. 10, 2024, a study, led by the University of Edinburgh, was the first to explore the…

On Sep. 10, 2024, researchers at the Karolinska Institute in Sweden announced a study that shows that gene…
On Sep. 9, 2024, a Princeton University team announced they had developed a way to probe chromosomes and…

On Sep. 9, 2024, Santo Fortunato, a professor at Luddy School of Informatics, reported that an international research…
On Sep. 6, 2024, researchers from the Wellcome Sanger Institute, University College London (UCL), and the University of…

On Sept. 5, 2024, the U.S. Department of Justice announced it had awarded nearly $8 million to cities,…

On Sep. 4, 2024, researchers at the University of Copenhagen announced the discovery of a gene that has…

On Sep. 4, 2024, an international consortium led by scientists from Uppsala University in Sweden reported that the…

On Sep. 4, 2024, researcher led by the Centers for Disease Control and Prevention (CDC) and Vanderbilt University…
On Sept. 3, 2024, a major study led by the UC Davis Comprehensive Cancer Center (UC DAVIS) has…
On Sept. 3, 2024, researchers at NORC at the University of Chicago and George Washington University’s Milken Institute…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…

On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…

On Aug. 30, 2024, Novavax announced the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) had received Emergency Use…
On Aug. 30, 2024, the California Institute for Regenerative Medicine (CIRM) approved $67.5 million to support five projects…
On Aug. 30, 2024, the United Nations (UN) announced that polio vaccination will begin for some 640,000 children…
On Aug. 29, 2024, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…
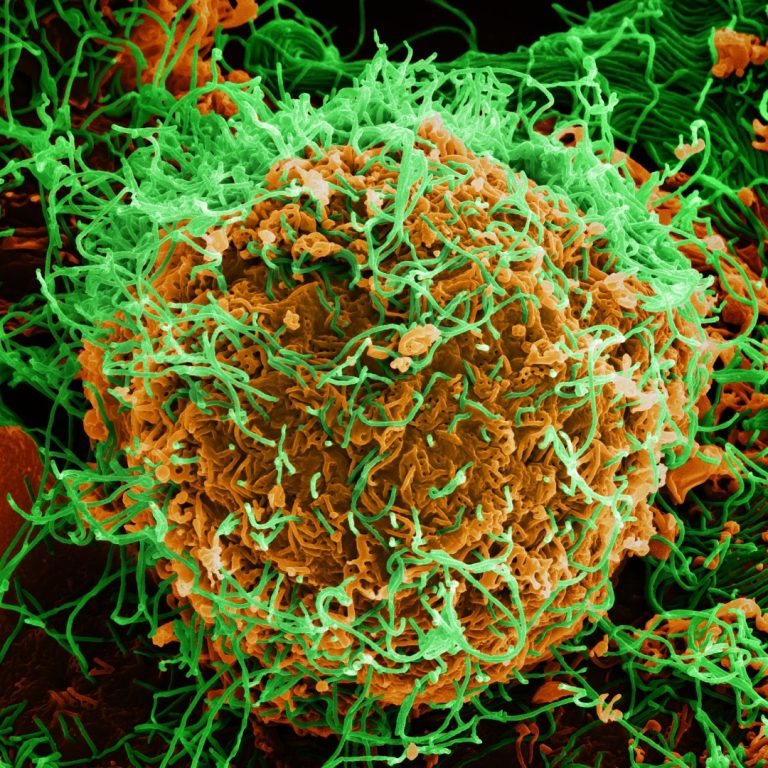
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…

On Aug. 28, 2024, the University of Würzburg announced research using newly generated “optogenetic” tobacco plants to investigated…

On Aug. 28, 2024, survey data from the Annenberg Public Policy Center (APPC) found that the number of…

On Aug. 28, 2024, Northwestern University and University of Texas-Southwestern announced results of a study that found bacterial…