Mariana Trench deep sea fish all have the same unique mutations
On Apr. 28, 2025, deep-sea fish adapt to some of the most extreme conditions on Earth. A study,…
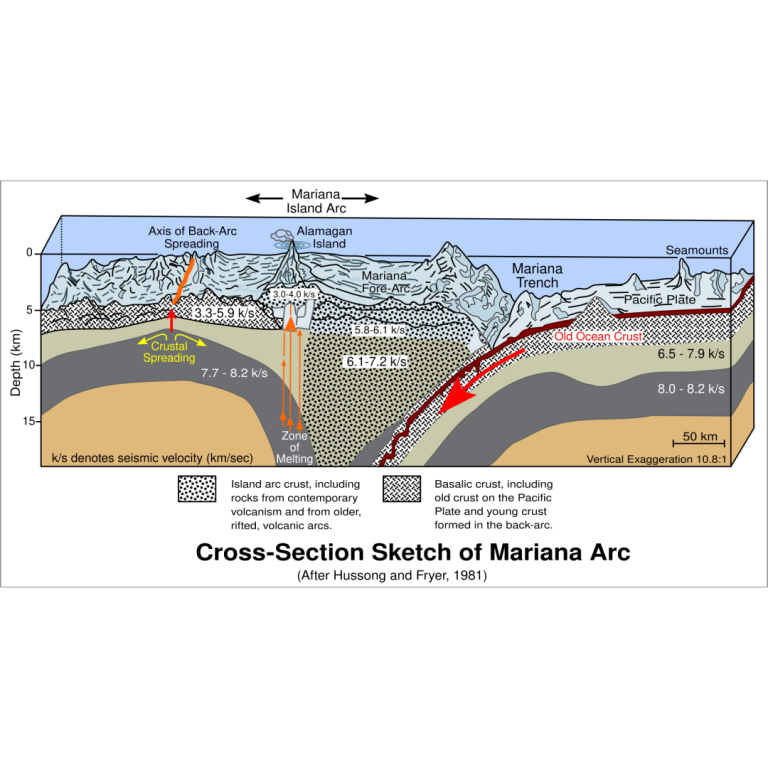
On Apr. 28, 2025, deep-sea fish adapt to some of the most extreme conditions on Earth. A study,…

On Apr. 28, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced it had awarded a more than $3…
On Apr. 26, 2025, the World Health Organization (WHO) reported that Uganda has declared the end of the…
On Apr. 25, 2025, the Texas Department of State Health Services (DSHS) reported that 646 cases of measles…

On Apr. 25, 2025, Amgen announced a $900 million expansion of its Ohio biotech manufacturing facility in New…

On Apr. 25, 2025, University of Washington announced that the Memory & Brain Wellness Center at Harborview Medical…

On Apr. 24, 2025, Thermo Fisher Scientific announced it will will invest an additional $2 billion in the…

On Apr. 23, 2025, researchers from the Wellcome Sanger Institute, University of California San Diego and their collaborators…

On Apr. 23, 2025, a Washington State University researcher was reported among a select few scientists who unveiled…
On Apr. 22, 2025, Seattle & King County was notified of a confirmed measles case in a King…

On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…

On Apr. 22, 2025, Syngenta announced that its next-generation insecticide Sovrenta® has received pre-qualification by World Health Organization…

On Apr. 22, 2025, Regeneron Pharmaceuticals announced a significant expansion of its manufacturing capacity through a new agreement…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Apr. 19, 2025, the Louisiana Department of Health (LDH) confirmed one case of measles in an adult…

On Apr. 19, 2025, the Virginia Department of Health (VDH) reported the state’s first measles case of the…
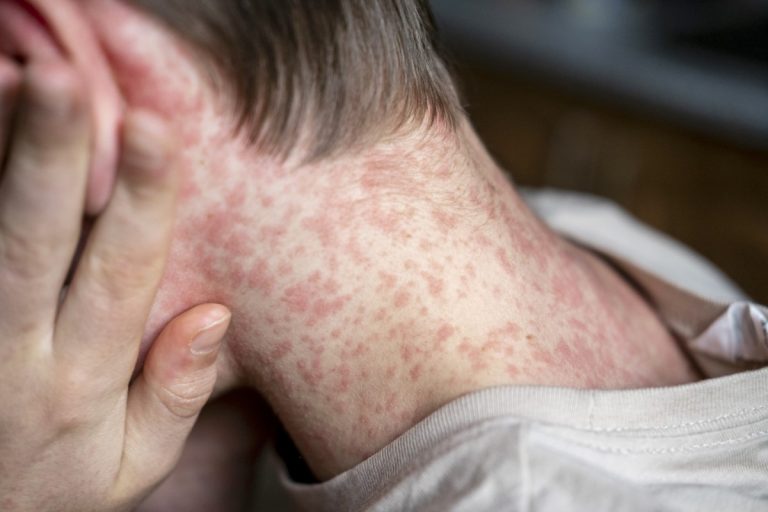
On Apr. 18, 2025, the Texas Department of State Health Services (DSHS) reported that 597 cases of measles…
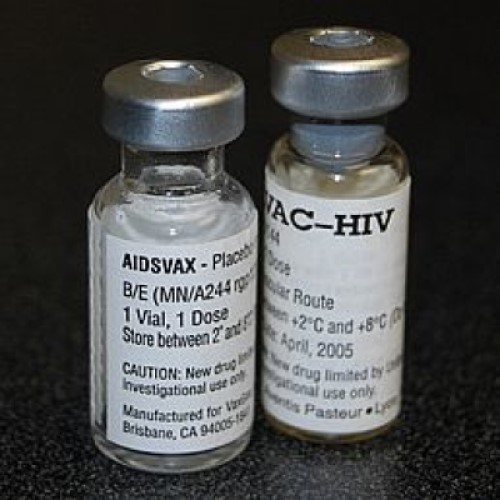
On Apr. 17, 2025, a study was published that shows the United States spent roughly US$12 billion on…

On Apr. 17, 2025, research-integrity analysts are warning that ‘journal snatchers’ — companies that acquire scholarly journals from…

On Apr. 17, 2025, the U.S. Centers for Disease Control and Prevention reported results from a study by…
On Apr. 17, 2025, researchers from the University of of Pittsburgh announced they have produced the most detailed…

On Apr. 17, 2025, the Department of Public Health and Human Services (DPHHS) and the Gallatin City-County Health…

On Apr. 17, 2025, The Tennessee Department of Health confirmed two additional confirmed cases of measles in middle…

On Apr. 16, 2025, A federal panel of medical experts has recommended an expansion of RSV vaccinations for…
On Apr. 16, 2025, The Sabin Vaccine Institute announces the launch of a multi-site Phase 2 clinical trial…

On Apr. 16, 2025, a study by Texas A&M showed chicken fertility rates in U.S. broiler eggs could…

On Apr. 15, 2025, the New Mexico Department of Health (NMDOH) reports an unvaccinated child in Doña Ana…

On Apr. 15, 2025, Moderna announced that its manufacturing facility in Harwell, Oxfordshire, the Moderna Innovation and Technology…

On Apr. 15, 2025, Novavax announced preliminary results from the SHIELD-Utah study (Study of Healthcare Workers and First…