UC Berkeley launched pop-up lab to monitor Bay Area sewage for COVID-19
On Oct. 29, 2020, the University of California, Berkeley (UC Berkeley) announced that it had launched a new…

On Oct. 29, 2020, the University of California, Berkeley (UC Berkeley) announced that it had launched a new…

On Oct. 28, 2020, researchers at the NIH announced that they had discovered a biological pathway that the…

On Oct. 27, 2020, researchers at Yale University and the Broad Institute of MIT and Harvard screened hundred…

On Aug. 27, 2020, immunologists at Scripps Research, in partnership with colleagues at the IAVI Neutralizing Antibody Center,…

On Oct. 26, 2020, Taconic Biosciences announced immediate humanized ACE2 mouse model availability for COVID-19 research. The SARS-CoV-2…

On Oct. 26, 2020, Membrion announced a breakthrough in the fight against the COVID-19 virus: a molecular coating…

On Oct. 23, 2020, Entos Pharmaceuticals announced it was receiving advisory services and funding of up to $5…

On Oct. 23, 2020, the U.S. FDA authorised a restart of clinical trials of the ChAdOx1 nCov-2019 Oxford…

On Oct. 23, 2020, AstraZeneca announced that the clinical trials for the AstraZeneca Oxford coronavirus vaccine, AZD1222, had…
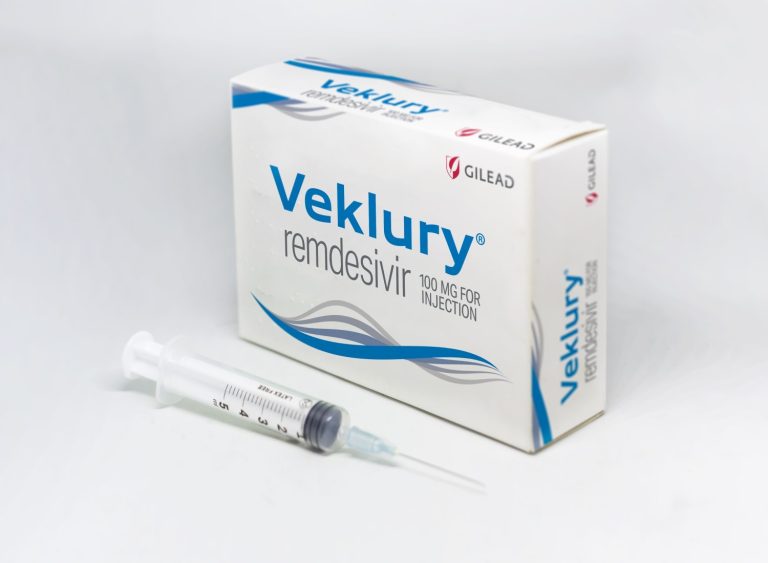
On Oct. 22, 2020, Gilead Sciences announced that the FDA had approved the antiviral drug Veklury (remdesivir) for…

On Oct. 21, 2020, ImmunityBio and NantKwest announced that the first patient had been dosed in the Phase…

On Oct. 20, 2020, the European Investment Bank (EIB) and Immunic announced the signing of a タ24.5 million…

On Oct. 20, 2020, researchers at Oregon Health & Science University (OHSU) and Oregon State University (OSU) reported…

On Oct. 19, 2020, Mateon Therapeutics announced the receipt of approval from Republica Argentina Poder Ejecutivo Nacional, the…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 15, 2020, ImmunityBio announced it had received authorization from the FDA to begin a Phase I…

On Oct. 15, 2020, Tonix Pharmaceuticals announced that the first participant was enrolled in the observational PRECISION study…

On Oct. 14, 2020, the U.S. Department of Health and Human Services (HHS) and Department of Defense (DOD)…
On Oct. 14, 2020, Regeneron announced that the FDA had approved Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn) for the…
On Oct. 14, 2020, the FDA approved Regeneron Pharmaceutical’s Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), a mixture of three…

On Oct. 13, 2020, Roche announced that it intended to launch a high-volume SARS-CoV-2 Antigen test as an…
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…

On Oct. 8, 2020, Emergent BioSolutions announced the initiation of the Phase 3 clinical trial that will evaluate…
On Oct. 7, 2020, Sorrento Therapeutics announced that it had discovered a small molecule termed Salicyn-30 that demonstrated…

On Oct. 6, 2020, NIH researchers announced they had found that a carbohydrate called heparan sulfate, which is…

On Oct. 5, 2020, the FDA awarded a research contract to the Stanford University School of Medicine to…

On Oct. 5, 2020, the Nobel Prize in Medicine 2020 was awarded to Harvey J. Alter, Michael Houghton…

On Oct. 5, 2020, the CDC announced the existence of published reports that showed limited, uncommon circumstances where…

On Oct. 1, 2020, widespread accurate testing for SARS-CoV-2, the virus that causes COVID-19, is one critical aspect…