Halted NIH Clinical Trials List Reveals Slashed Treatments for Cancer, COVID and Minority Health
On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…

On Oct. 30, 2025, the California Institute for Regenerative Medicine (CIRM) approved awarding $27 million through three grants…

On Feb. 13, 2024, CRISPR Therapeutics announced that the European Commission had granted conditional marketing authorization to CASGEVY…
On Dec. 8, 2023, the U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia,…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…

On Nov. 16, 2023, Vertex and CRISPR Therapeutics announced that the United Kingdom (U.K.) Medicines and Healthcare products…

Our Milestones in CRISPR cartoon illustrates several significant achievements in the development of the technology with commentary by…
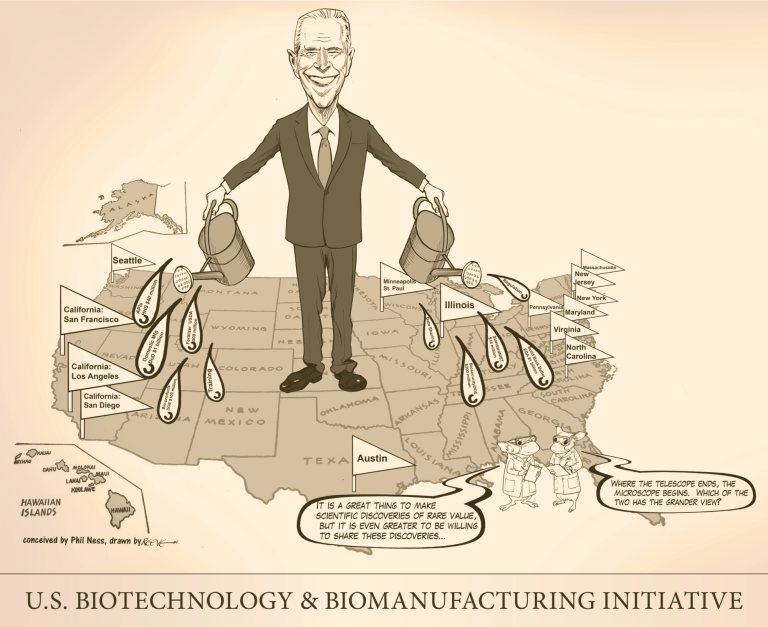
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…

The Evolution of CRISPR illustrates its climb out of the primordial ocean on the backs of eukaryotic cells…
On Feb. 17, 2021, Novartis announced that it had entered into a grant agreement with the Bill &…

On Jan. 11, 2021, Editas Medicine announced the U.S. Food and Drug Administration (FDA) had cleared the initiation…

On Dec.14, 2020, the US Food and Drug Administration (FDA) announced they had granted Investigational New Drug (IND)…

On May 15, 2020, the California Institute for Regenerative Medicine (CIRM) board approved new clinical trials for COVID-19…
On Feb. 25, 2020, the U. S. National Institutes of Health (NIH) announced it had launched a $1…

On Dec. 2, 2019, the U.S. Food and Drug Administration (FDA) announced it had approved the drug crizanlizumab…

On Jul. 19, 2019, Victoria Gray, 34, of Forest, Miss., became the first patient ever to be publicly…

On Jun. 7, 2017 the FDA approved Endari (L-glutamine oral powder) for patients age five years and older…

In 2008, the St.ᅠ Jude Children’s Research Hospital received National Cancer Institute (NCI) comprehensive center designation. St.ᅠJude Children’s…
In 2001, The J.P. McCarthy Cord Stem Cell Bank at the Karmanos Cancer Institute was founded as a…

On Aug. 9, 1993, Francisco Mojica published “Transcription at different salinities of Haloferax mediterranei sequences adjacent to partially…

On Feb. 27, 1992, Dr. Robert Adams and Dr. Virgil C. McKie of the Medical College of Georgia…

In 1986, Dr. Marilyn Hughes Gaston while deputy branch chief of the Sickle Cell Disease Branch at the…

On Sept. 21, 1983, Sickle Cell Month was officially recognized nationally when the U.S. House of Representatives unanimously…

In 1977, St.ᅠ Jude Children’s Research Hospital received National Cancer Institute designation. St.ᅠJude Children’s Research Hospital opened it’s…

In 1975, Sickle Cell Disease Awareness Month originated when the Sickle Cell Disease Association of America and its…

On Feb. 4, 1962, St.ᅠ Jude Children’s Research Hospital opened it’s doors.This was the day that Danny Thomas…

In 1923, Dr. Virgil P. Sydenstricker published the first documented case of sickle cell disease, with full autopsy…