Early mutation in SARS-CoV-2 virus in Europe led to its domination world wide
On Nov. 12, 2020, a study published in Science by a team of researchers in the U.S. and…
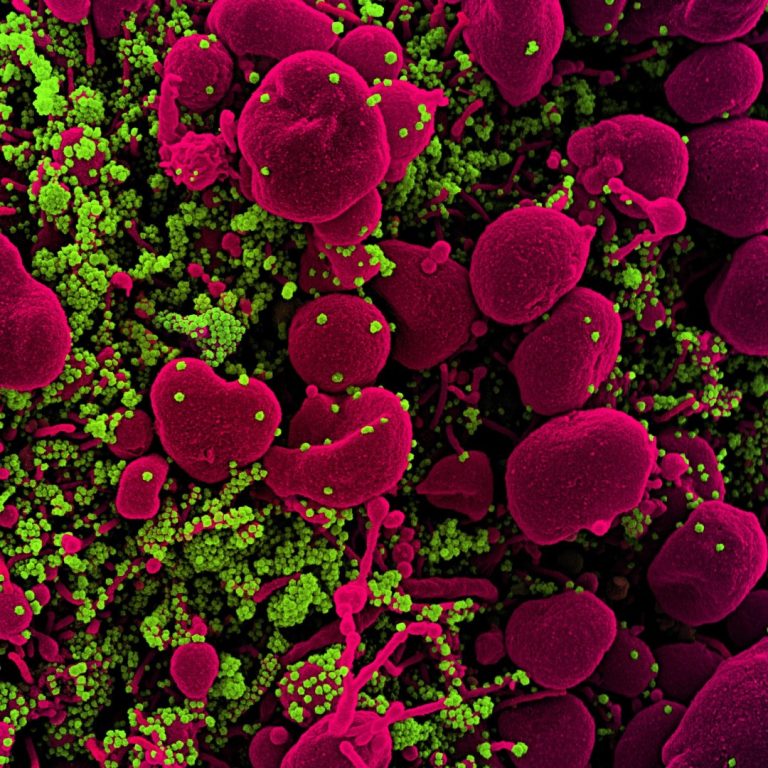
On Nov. 12, 2020, a study published in Science by a team of researchers in the U.S. and…
On Nov. 11, 2020, Sorrento Therapeutics announced that it was filing an investigational new drug application (IND) for…

On Nov. 11, 2020, National Institute of Allergy and Infectious Diseases (NIAID) Director Anthony S. Fauci, M.D., and…

On Nov. 11, 2020, Howard Hughes Medical Institute Investigator Michel Nussenzweig and his team reported that people who…

On Nov. 11, 2020, Pfizer and BioNTech announced that they had reached an agreement with the European Commission…

On Nov. 10, 2020, Oxford Immunotec announced the release of data from a prospective cohort study in keyworkers…

On Nov. 19, 2020, Medigen Vaccine Biologics and BlueWillow Biologics announced a partnership to develop S-2P-NE-01, a nasal…

On Nov. 10, 2020, 3M announced that its TB Quat Disinfectant Ready-to-Use Cleaner had been approved by the…
On Nov. 10, 2020, BD (Becton, Dickinson) announced its rapid, point-of-care, SARS-CoV-2 antigen test for use on the…

On Nov. 9, 2020, PerkinElmer announced that EUROIMMUN, a PerkinElmer company, had launched the Anti-SARS-CoV-2 QuantiVacTM ELISA (IgG)…
On Nov. 9, 2020, Pfizer and BioNTech announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 had demonstrated evidence…

On Nov. 9, 2020, Corvus Pharmaceuticals announced that it had completed patient enrollment in its Phase 1 study…

On Nov. 9, 2020, the Fred Hutchinson Cancer Research Center announced the start of volunteer enrollment for a…

On Nov. 8, 2020, research from the U.S. had shown that white-tailed deer were being infected with SARS-CoV-2,…

On Nov. 8, 2020, ImmunityBio announced positive study results for their human Ad5 (hAd5) COVID-19 vaccine candidate, which…

On Nov. 6, 2020, the FDA authorized the first serology test that detected neutralizing antibodies from recent or…
On Nov. 6, 2020, Mesa Biotech announced it had been awarded a contract up to $13 million from…

On Nov. 5, 2020, Neoleukin Therapeutics announced the publication in Science of research describing novel molecules designed to…

On Nov. 4, 2020, the University of Oxford announced that its rapid COVID-19 test was being used at…

On Nov. 3, 2020, OraSure Technologies announced its DNA Genotek subsidiary has received Emergency Use Authorization from the…

On Nov. 2, 2020, CureVac announced positive interim data from its ongoing Phase 1 dose-escalation study evaluating the…

On Nov. 2, 2020, ACON Laboratories announced the launch of its Flowflexル SARS-COV-2 Antigen Rapid Test. The Flowflex…
On Nov. 1, 2020, scientists announced that increasing evidence shows that both natural and synthetic antimicrobial peptides (AMPs),…

On Oct. 31, 2020, the U.S. Department of Health and Human Services and the U.S. Department of Defense…

On Oct. 30, 2020, Innovation Pharma and George Mason University jointly announced completion of extensive laboratory testing supporting…

On Oct. 30, 2020, Icosavax announced the launch of the companyメs COVID-19 vaccine program with preclinical data on…

On Oct. 30, 2020, the U.S. Department of Health and Human Services (HHS) and the U.S. Department of…

On Oct. 30, 2020 InBios announced that it had received Emergency Use Authorization from the U.S. Food and…
On Oct. 29, 2020, Mammoth Biosciences announced that it had signed agreements with MilliporeSigma and Hamilton Company targeting…

On Oct. 29, 2020, OncoSec Medical announced that the FDA had approved the Investigational New Drug (IND) application…