CSL injects $2.3b into US for valuable blood plasma
On Nov. 18, 2025, CSL, Australia’s largest biotech company announced plans to expand its US presence with a…

On Nov. 18, 2025, CSL, Australia’s largest biotech company announced plans to expand its US presence with a…

On Nov. 15, 2024, the AARP, the Alzheimer’s Disease Data Initiative (AD Data Initiative), and the Institute for…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…
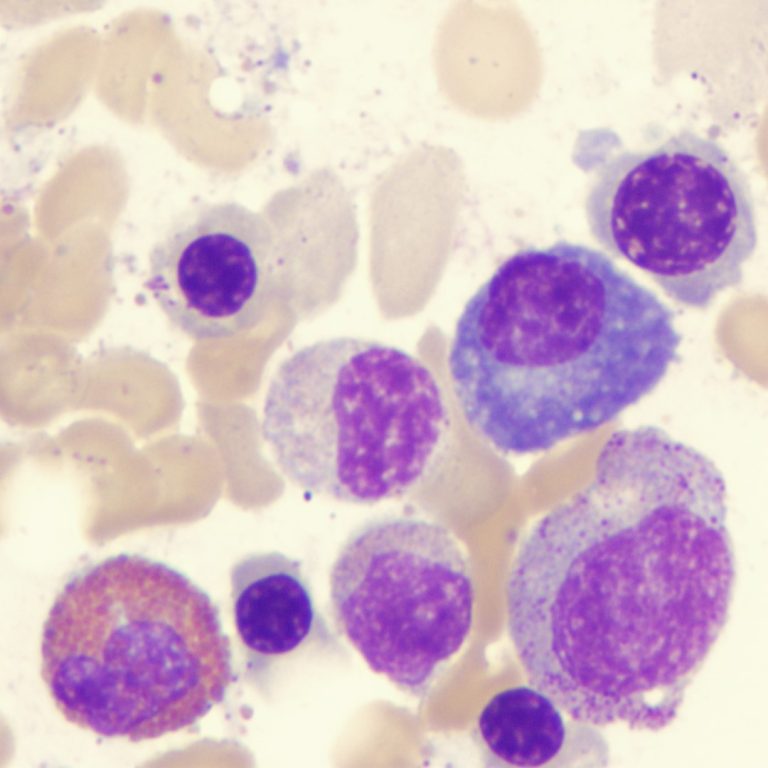
On Aug. 19, 2024, an international team of scientists led by researchers from the Translational Genomics Research Institute (TGen) announced…

On Jul. 25, 2024, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Jan. 13, 2023, a team of researchers including faculty at the Translational Genomics Research Institute (TGen), part…

On Feb. 23, 2022, Agilent Technologies announced that researchers at the Indian Institute of Technology Bombay in India…

On Dec. 6, 2021, the World Health Organization Guideline Development Group of international experts announced in the BMJ…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Aug. 19, 2021, Oregon Health & Science University (OHSU) announced that it was part of a randomized,…

On Aug. 18, 2021, Agilent Technologies announced that researchers at the Indian Institute of Technology Bombay in India…

On Aug. 18, 2021, the final results of the Clinical Trial of COVID-19 Convalescent Plasma in Outpatients (C3PO)…

On Aug. 16, 2021, ADMA Biologics announced that it had received U.S. Food and Drug Administration approval for…

On Mar. 2, 2021, the NIH halted a clinical trial evaluating the safety and effectiveness of COVID-19 convalescent…

On Feb. 18, 2021, Agilent Technologies announced the launch of the Agilent Dako SARS-CoV-2 IgG Enzyme-Linked Immunosorbent Assay…

On Jan. 15, 2021, Oxford University announced that on advice of the independent Data Monitoring Committee (DMC), recruitment…

On Jan. 12, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…

On Jan. 11, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…
On Dec. 17, 2020, ACON Laboratories announced that its SARS-COV-2 IgG/IgM Rapid Test has been authorized for emergency…

On Oct. 19, 2020, Bio-Techne announced that ProteinSimple, a Bio-Techne brand, released its SARS-CoV-2 Multi-Antigen Serology Module for…

On Oct. 8, 2020, Emergent BioSolutions announced the initiation of the Phase 3 clinical trial that will evaluate…

On Sept. 22, 2020, the NIH annunced that two randomized, placebo-controlled clinical trials expanded enrollment to further evaluate…
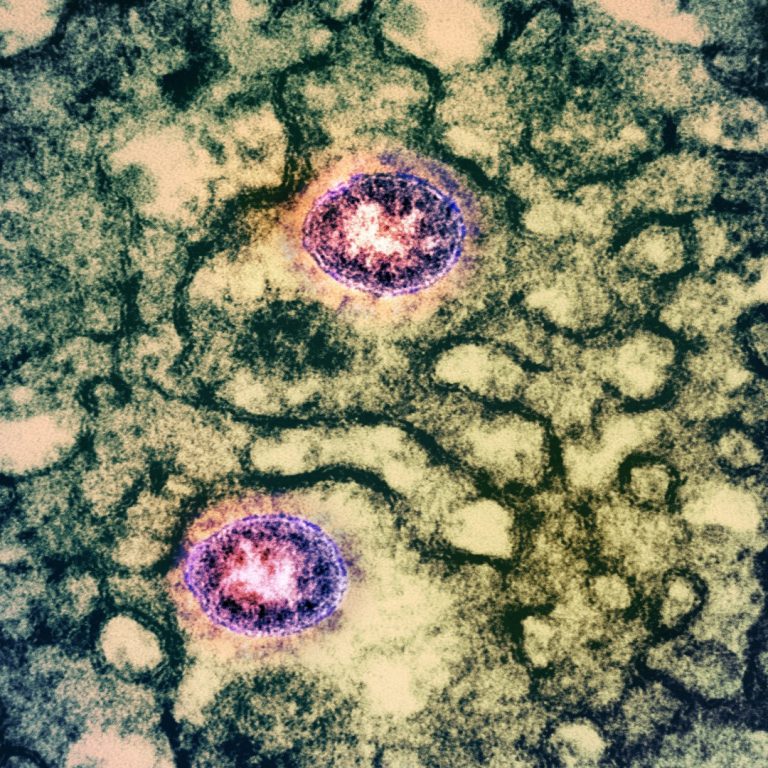
On Sept. 16, 2020, AXIM Biotechnologies announced that it had filed an Emergency Use Authorization (EUA) application with…

On Sept. 3, 2020, ADMA Biologics announced the launch of COVID-19 ImmunoRank Neutralization MICRO-ELISA, a proprietary, fully-validated ELISA…

On Aug. 28, 2020, the U.S. Dept. of Veterans Affairs (VA) announced a new clinical trial to study…

On Aug. 24, 2020, XBiotech announced that the U.S. Food and Drug Administration (FDA) issued an emergency use…

On Aug. 23, 2020, the FDA issued an emergency use authorization (EUA) for investigational convalescent plasma for the…

On Aug. 23, 2020, the Mayo Clinic announced that with the FDA Emergency Use Authorization of convalescent plasma,…