Oklahoma Children’s Hospital Takes Swift Action Against Measles
On Apr. 2, 2025, the Oklahoma Children’s Hospital (OU Health) announced it had taken swift action to address…

On Apr. 2, 2025, the Oklahoma Children’s Hospital (OU Health) announced it had taken swift action to address…

On Apr. 1, 2025, the Tennessee Department of Health confirmed three additional cases of measles in Middle Tennessee…

On Mar. 28, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Mar. 27, 2025, The Hospital for Sick Children (SickKids) announced it is harnessing the transformative potential of…

On Mar. 27, 2025, the Louisiana Office of the Surgeon General reported on Facebook that two infants had…
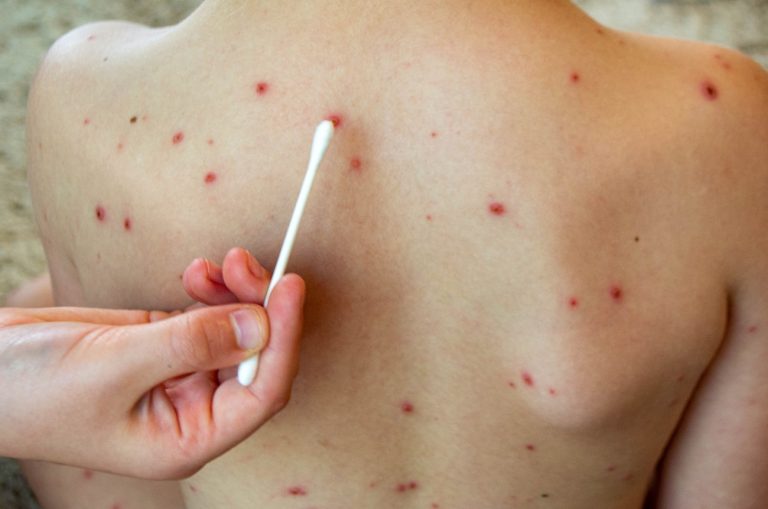
On Mar. 26, 2025, the Kansas Department of Health and Environment (KDHE) reported 23 positive cases of measles…

On Mar. 25, 2025, Ohio Department of Health (ODH) announced a measles outbreak in Ashtabula County and one…

On Mar. 25, 2025, the Oklahoma State Department of Health (OSDH) reported that nine additional measles cases had…

On Mar. 21, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…
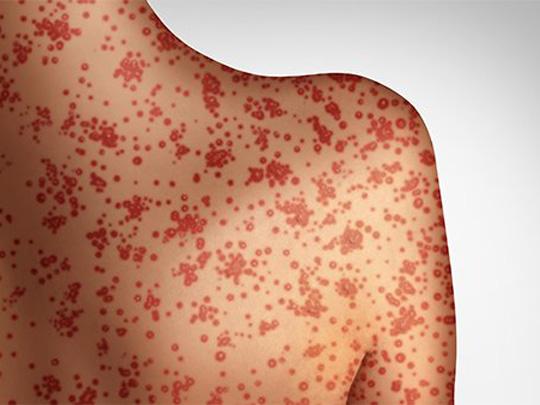
On Mar. 21 , 2025, the Tennessee Department of Health confirmed the state’s first measles case in 2025…
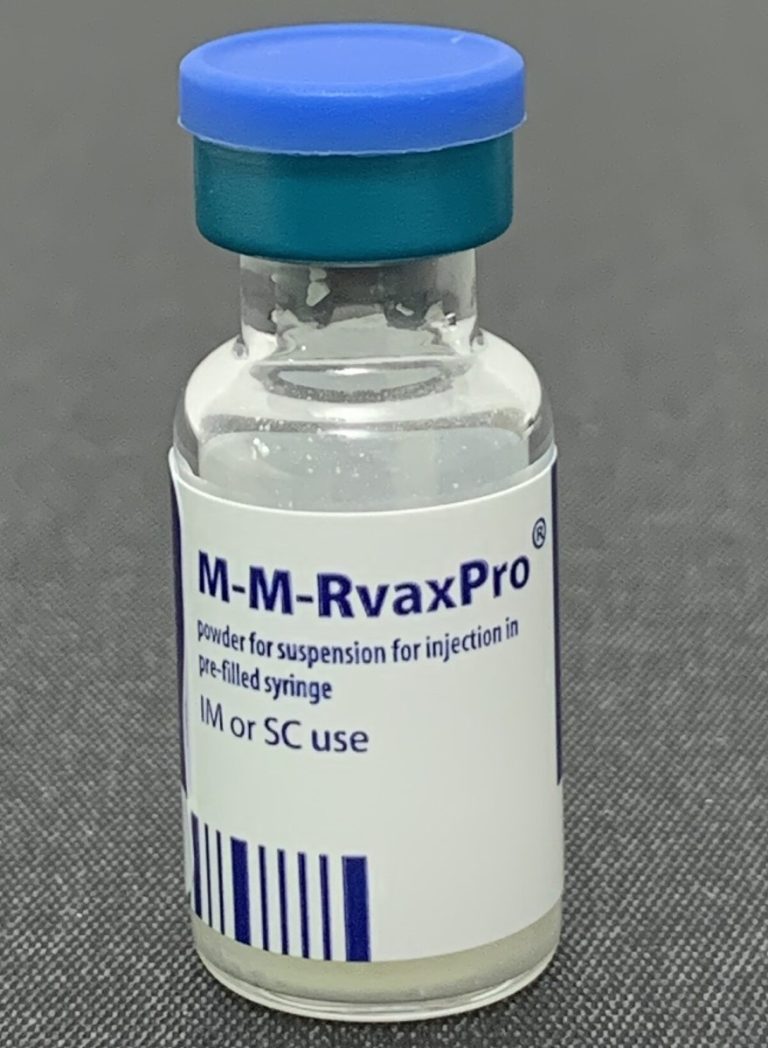
On Mar. 20, 2025, Ohio Department of Health (ODH) reported the state’s first measles case of 2025. The…

On Mar. 14, 2025, the Michigan Department of Health and Human Services (MDHHS) and Oakland County Health Division…

On Mar. 14, 2025, the Oklahoma State Department of Health (OSDH) received notification of two more probable measles…

On Mar. 13, 2025, The Kansas Department of Health and Environment (KDHE) and the Stevens County Health Department…

On Mar. 11, 2025, the New York State Department of Health today announced the first case of measles…

On Mar. 11, 2025, in an excerpt from his book Booster Shots (Penguin Random House), pediatrician and infectious…

On Mar. 11, 2025, the Oklahoma State Department of Health (OSDH) reported the first two measles cases in…

On Mar. 7, 2025, the Texas Department of State Health Services reported updated information about an outbreak of…

On Feb. 26, 2025, the Texas Department of State Health Services reported the first death from measles in…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…

On Feb. 13, 2025, Ralph L. Abraham, MD, the Louisiana Surgeon General announced that the state will no…

On Jan. 30, 2025, the California Institute for Regenerative Medicine (CIRM) announced it has awarded nearly $100 million…

On Dec. 20, 2024, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) had approved Alhemo®…

On Dec. 16, 2024, a study published in BMC Cardiovascular Disorders shows that pediatric and young adult COVID-19…
On Nov. 21, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding $29.7 million to…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…
On Aug. 6, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved vorasidenib (Voranigo, Servier…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…