Kentucky Health Officials Confirm 2 Whooping Cough Deaths in Young Infants
On Jun. 6, 2025, officials with the Kentucky Department for Public Health (KDPH) announced that two infants have…

On Jun. 6, 2025, officials with the Kentucky Department for Public Health (KDPH) announced that two infants have…
On May 30, 2025, the Texas Department of State Health Services (DSHS) reported 738 cases of measles have…
On May, 27, 2025, an Ohio State University (OSU) study has revealed the biological secret to the Zika…
On May 23, 2025, the Texas Department of State Health Services (DSHS) reported 728 cases of measles have…
On May 16, 2025, the Texas Department of State Health Services (DSHS) reported 718 cases of measles have…

On May 15, 2025, the World Health Organization (WHO) announced it has published its World health statistics report…

On May 15, 2025, a team at Children’s Hospital of Philadelphia (CHOP) and Penn Medicine announced that a…
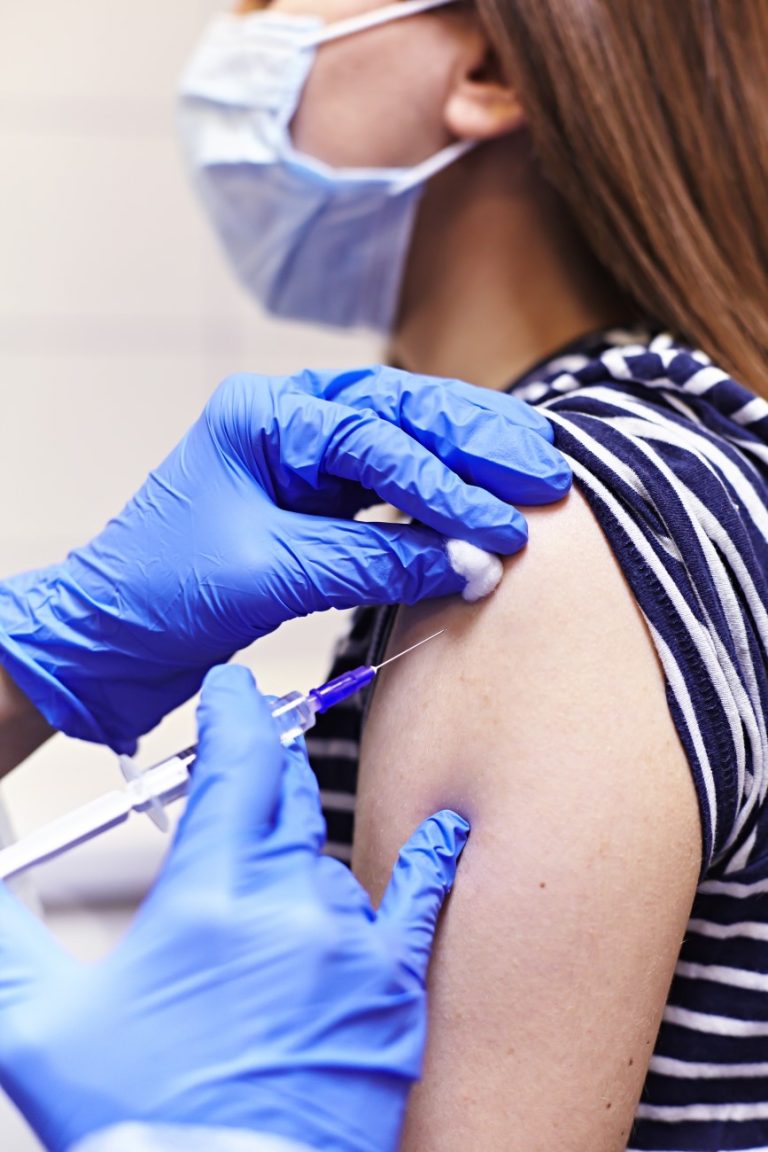
On May 9, 2025, the Texas Department of State Health Services (DSHS) reported 709 cases of measles have…

On May 6, 2025, UT Southwestern Medical Center and Children’s Health℠ announced a historic nine-figure grant from the Moody Foundation…

On May 2, 2025, the Louisiana Department of Health (LDH) reported an increase in whooping cough (pertussis) cases…

On May 2, 2025, the Centers for Disease Control reported twelve pediatric deaths associated with seasonal influenza virus…

On May 2, 2025, the Texas Department of State Health Services (DSHS) reported 683 cases of measles have…
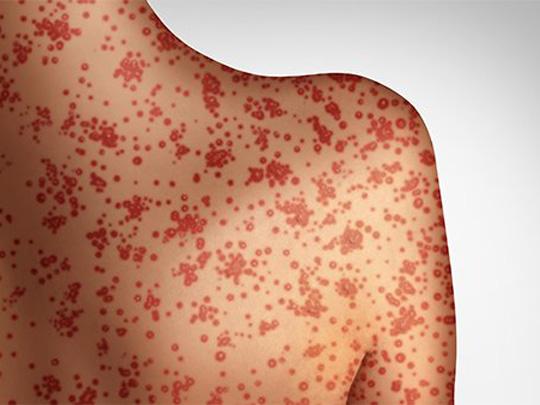
On Apr. 25, 2025, the Texas Department of State Health Services (DSHS) reported that 646 cases of measles…

On Apr. 19, 2025, the Louisiana Department of Health (LDH) confirmed one case of measles in an adult…
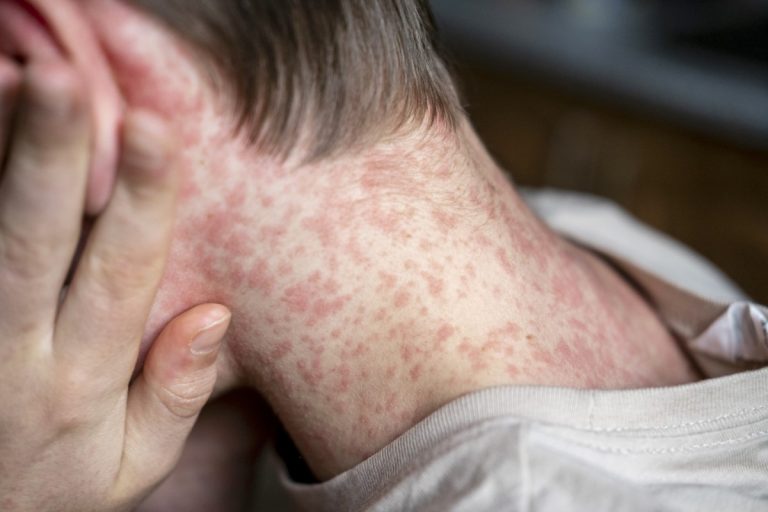
On Apr. 18, 2025, the Texas Department of State Health Services (DSHS) reported that 597 cases of measles…
On Apr. 17, 2025, a study was published that shows the United States spent roughly US$12 billion on…

On Apr. 17, 2025, The Tennessee Department of Health confirmed two additional confirmed cases of measles in middle…
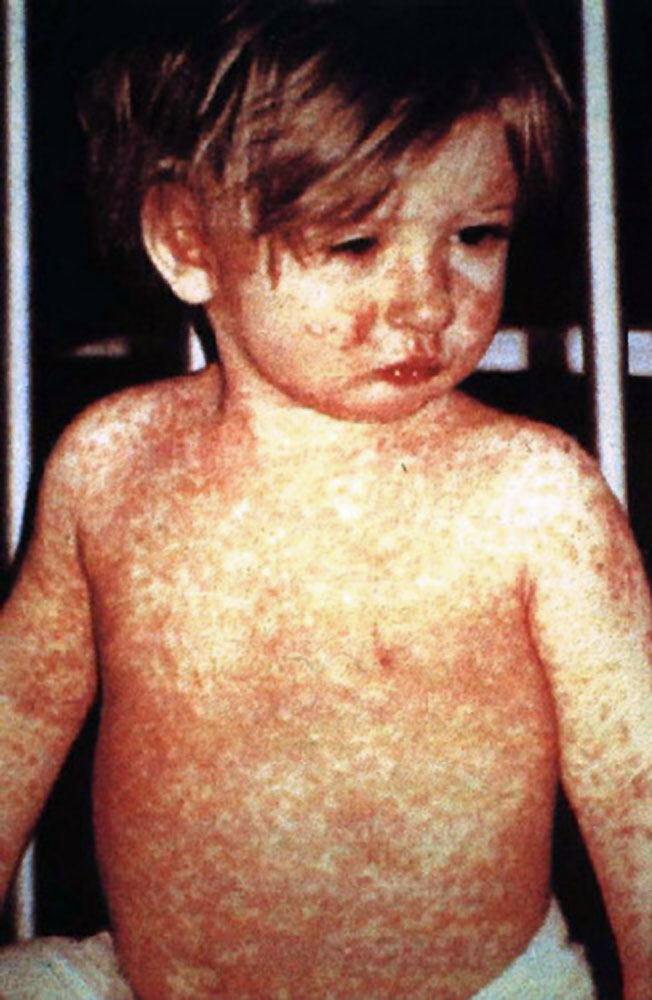
On Apr. 11, 2025, the Texas Department of State Health Services (Texas DSHS) reported that 541 cases of…

On Apr. 8, 2025, the Hawaiʻi Department of Health (DOH) State Laboratories Division confirmed a case of measles…

On Apr. 7, 2025, The Indiana Department of Health (IDOH) reported the first laboratory confirmed case of measles…

On Apr. 6, 2025, the Texas Department of State Health Services has reported the second measles death of…

On Apr. 4, 2025, a 3-year-old girl from the western state of Durango is Mexico’s first confirmed human…

On Apr. 4, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On Apr. 2, 2025, Northwestern University engineers announced they have developed a pacemaker so tiny that it can…

On Apr. 2, 2025, the Oklahoma Children’s Hospital (OU Health) announced it had taken swift action to address…

On Apr. 1, 2025, the Tennessee Department of Health confirmed three additional cases of measles in Middle Tennessee…

On Mar. 28, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Mar. 27, 2025, The Hospital for Sick Children (SickKids) announced it is harnessing the transformative potential of…

On Mar. 27, 2025, the Louisiana Office of the Surgeon General reported on Facebook that two infants had…