WHO reported more than half the world faces high measles risk
On Feb. 21, 2024, the World Health Organization (WHO) reported that more than half the world’s countries will…
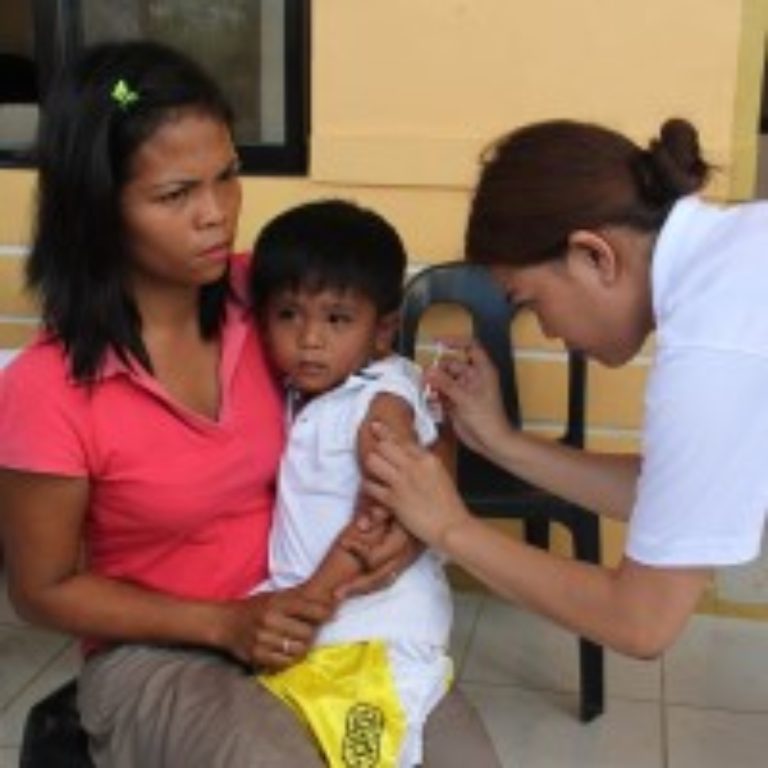
On Feb. 21, 2024, the World Health Organization (WHO) reported that more than half the world’s countries will…
On Feb. 14, 2024, the National Institute of Allergy and Infectious Diseases (NIAID) reported study results that showed…

On Feb. 6, 2024, Oregon Health & Science University (OHSU) announced research that revealed as much as a…
On Feb. 5, 2024, CSL and and Arcturus Therapeutics announced the results of a follow-up analysis of a…

On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…

On Dec. 19, 2023, COVAX, the multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020,…

On Dec. 18, 2023, COVID-19 vaccine maker BioNTech (22UAy.DE) announced that it aims to start production at its…

On Dec. 18, 2023, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…

On Dec. 13, 2023, National Institute of Allergy and Infectious Diseases (NIAID) scientists announced findings published in the…

On Dec. 6, 2023, the federal government announced it had expanded the Home Test to Treat program, an…

On Dec. 5, 2023, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid XBB.1.5 Vaccine (Recombinant…

On Dec. 4 2023, the University of Wisconsin-Madison researchers announced that while analyzing data from wastewater samples collected…

On Nov. 29, 2023, Novavax’s partner, SK bioscience, announced that Novavax’s updated COVID-19 vaccine (NVX-CoV2601) had received Emergency…

On Nov. 28, 2023, Novavax announced that Nuvaxovid XBB.1.5 COVID-19 Vaccine (NVX-CoV2601) had been granted Emergency Use Listing…

On Nov. 28, 2023, CSL and Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor and Welfare (MHLW)…
On Nov. 27, 2023, University of Wisconsin-Madison researchers released a study that showed COVID-19 caused an alarming surge…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…

On Nov. 16. 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Following years of…

On Nov. 13, 2023, the Journal JAMA reported evidence that showed COVID-19 had widened the gendered life expectancy…
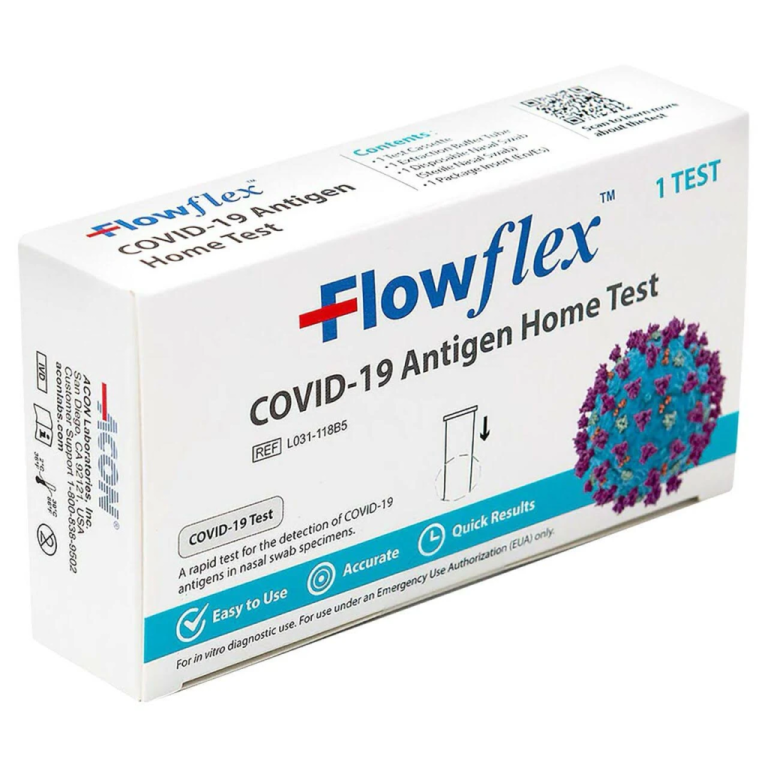
On Nov. 9, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC)…
On Nov. 6, 2023, researchers at NYU Grossman School of Medicine announced they had engineered the first mice…

On Oct. 31, 2023, Novavax announced that the European Commission had granted approval for Nuvaxovid XBB.1.5 dispersion for…

On Oct. 18, 2023, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Oct. 18, 2023, Novavax announced that Singapore’s Health Sciences Authority (HSA) had granted full approval for Novavax’s…

On Oct. 16. 2023, the U.S. Dept. of Health and Human Services reported results of an audit that…

On Oct. 3, 2023, Novavax announced that it’s COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) (NVX-CoV2601) had received Emergency Use…

On Oct. 3, 2023, Vir Biotechnology announced that the Biomedical Advanced Research and Development Authority (BARDA), part of…

On Oct. 3, 2023, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of…

On Oct. 2, 2023, the Nobel Prize in Physiology or medicine was awarded jointly to Katalin Kariko and…