Seattle Children’s researchers discovered that functional COVID-19 antibodies are lost quickly after mild cases
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…

On May 26, 2021, Henry Schein announced the Company was awarded a $53.4 million contract from the U.S….

On May 26, 2021, the National Center for Biotechnology Information announced a report that aimed to analyze the…

On May 26, 2021, GlaxoSmithKline and Vir Biotechnology announced the U.S. Food and Drug Administration (FDA) granted an…

On May 25, 2021, ImmunityBio announced two South African studies to examine the potential for using its hAd5…

On May 25, 2021, Moderna announced that the Phase 2/3 study of its COVID-19 vaccine (mRNA-1273) in adolescents…
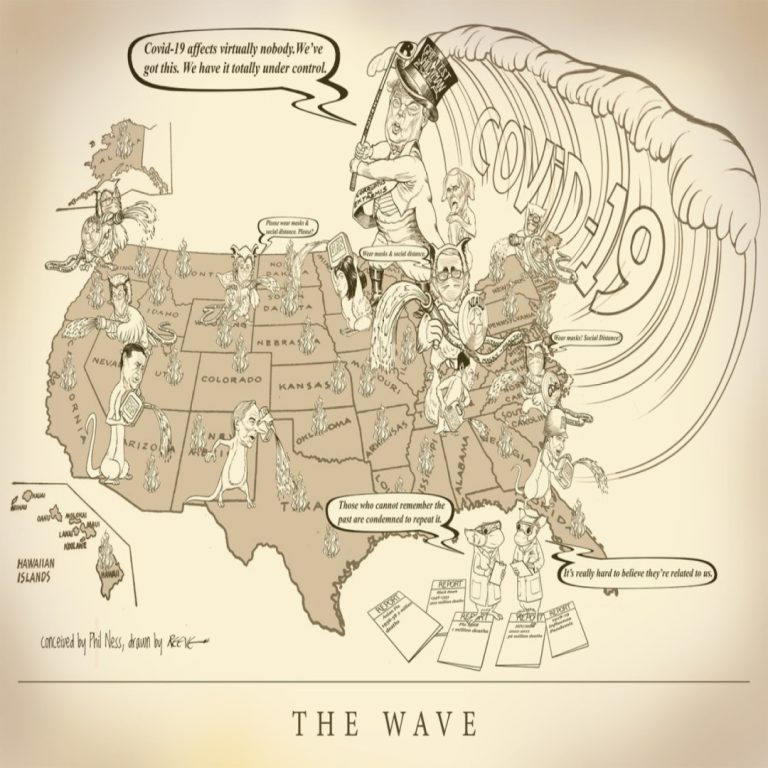
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…

On May 24, 2021, Moderna and Aldevron announced their expanded collaboration in support of the Moderna COVID-19 Vaccine…

On May 24, 2021, LabCorp reported that nearly 87% of naturally infected COVID-19 patients maintained antibodies to SARS-CoV-2…

On May 24, 2021, Pfizer announced that the first enrolled subjects had received their immunizations as part of…

On May 22, 2021, Moderna and Samsung Biologics announced a Manufacturing Services and Supply Agreement in which Samsung…

On May 22, 2021, Novavax announced the signing of a non-binding memorandum of understanding (MoU) with the Ministry…

On May 21, 2021, Moderna announced that the Ministry of Food and Drug Safety of South Korea (MFDS)…

On May 21, 2021, Novavax announced that the full results from the Phase 3, randomized, observer-blinded, placebo-controlled trial…

On May 21, 2021, Novavax announced participation in a mix-and-match (vaccine interchangeability) COVID-19 vaccine booster trial that led…

On May 21, 2021, AstraZeneca announced that it had been granted a special approval for emergency use in…

On May 21, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Johnson…

On May 21, 2021, world leaders the Global Health Summit adopted an agenda to overcome the COVID-19 pandemic,…

On May 21, 2021, Moderna announced that the Ministry of Health, Labour and Welfare (MHLW) of Japan granted…

On May 20, 2021, XPhyto announced that its distribution, storage and logistics partner, Max Pharm has begun the…

On May 20, 2021, Sorrento Therapeutics announced receipt of clearance from the Brazilian regulatory agency (ANVISA) to proceed…

On May 20, 2021, Pfizer and BioNTech announced a new agreement with the European Commission (EC) to supply…
On May 20, 2021, Pfizer and BioNTech announced an agreement with Turkeyメs Ministry of Health to supply 60…

On May 19, 2021, APEIRON Biologics announced the next development steps for APN01 (alunacedase alfa) for the treatment…

On May 18, 2021, GlaxoSmithKline and Medicago reported positive interim Phase 2 clinical trial safety and immunogenicity data…

On May 17, 2021, Oxford University and Oracle announced they had created a Global Pathogen Analysis System combining…

On May 17, 2021, Sanofi and GSK reported that their adjuvanted recombinant COVID-19 vaccine candidate achieved strong rates…

On May 13, 2021, CureVac announced together with its collaboration partner GSK, first preclinical data in a rat…

On May 12, 2021, Moderna announced a new supply agreement with the government of Australia for 25 million…

On May 12, 2021, Inovio Pharma announced that its next-generation Pan-COVID-19 vaccine candidate, INO-4802, induced potent neutralizing antibodies…