UCLA team discovers how to target ‘undruggable’ protein that fuels aggressive leukemia
On Dec. 9, 2025, researchers at the UCLA Health Jonsson Comprehensive Cancer Center announced they have identified a…
On Dec. 9, 2025, researchers at the UCLA Health Jonsson Comprehensive Cancer Center announced they have identified a…
On Dec. 8, 2025, a study led by Roswell Park Comprehensive Cancer Center reveals that a patient’s race…

On Nov. 4, 2025, the U.S. Food and Drug Administration (FDA) approved revumenib, a first-in-class oral menin inhibitor,…
On Oct. 13, 2025, University of Cambridge scientists announced they have used human stem cells to create three-dimensional…

On Sept. 25, 2025, Final data from a landmark clinical trial in patients with an aggressive form of…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…
On Aug. 5, 2024, Novartis and Viatris were named a federal lawsuit in Maryland by the family of…
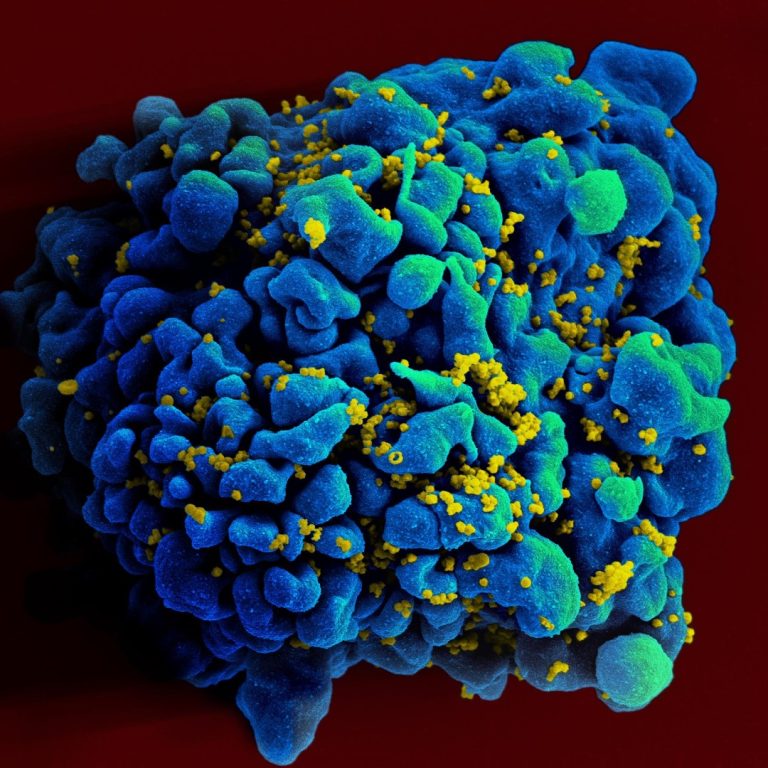
On Jul. 25, 2024, the World Health Organization (WHO) announced during the 25th International AIDS Conference, being held…

On Aug. 24, 2023, researchers at City of Hope announced were awarded $32.3 million from the California Institute…
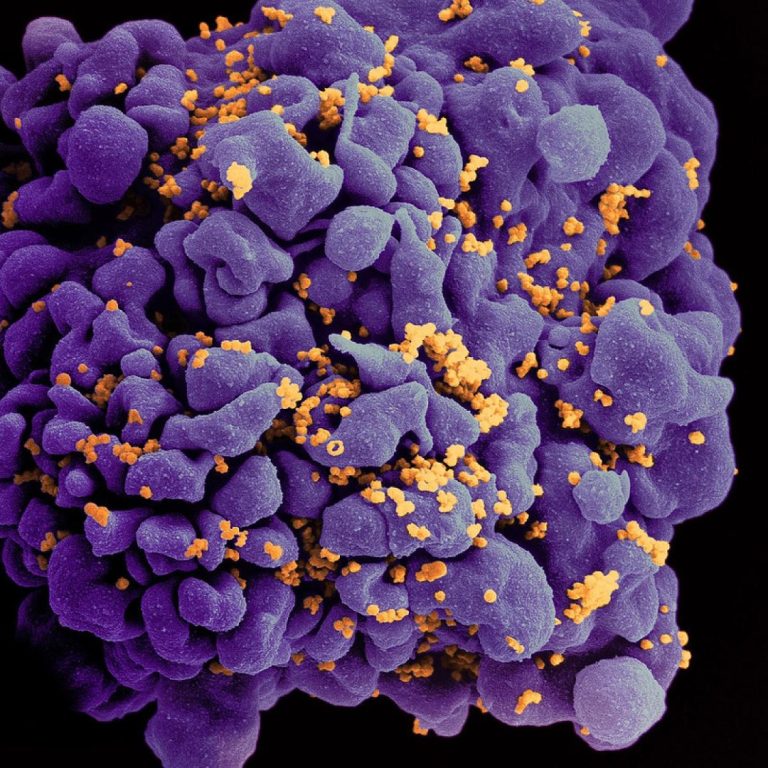
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…
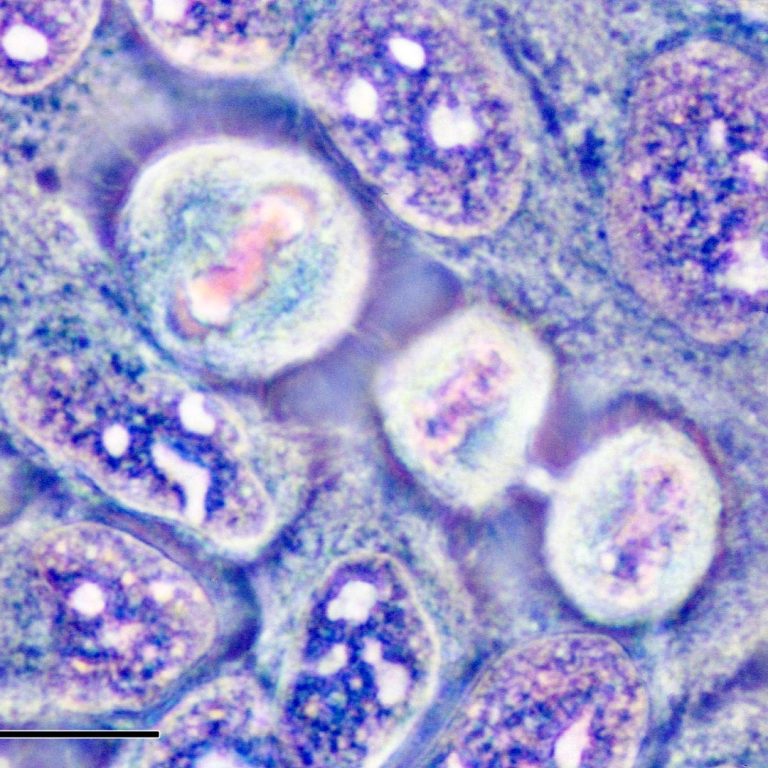
On Mar. 7, 2023, researchers at the National Institutes of Health reported the benefits of screening adult patients…
On Feb. 15, 2023, it was reported that a 53-year-old man in Germany had become at least the…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…

On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…

On Feb. 2, 2022, a new analysis of the first two patients treated in a clinical trial with…

On Oct. 13, 2021, the World Health Organization (WHO) honoured the late Henrietta Lacks with a WHO Director-General’s…

On Oct. 1, 2021, Kite, a Gilead Company, announced the U.S. Food and Drug Administration had granted approval…

On Apr. 21, 2020, the Janssen Pharmaceutical Companies of Johnson & Johnson announced U.S. Food and Drug Administration…

On Sept. 12, 2019, UPenn completed its exclusive, 7-year research and development alliance with Novartis, that spawned the…

On Aug. 21, 2019, the Siteman Cancer Center announced it was awarded a $15 million grant to better…

On Jan. 29, 2019, Ontario Institute for Cancer Research’s (OICR) Drug Discovery Program’s first-of-its-kind leukemia therapy discovery received…

On Nov. 28, 2018, The U.S. Food and Drug Administration (FDA) announced it had approved the first ever…

On Mar. 9, 2018, Boston-based cancer company Partner Therapeutics announced that it had acquired the global rights to…

On Aug. 30, 2017, the U.S. Food and Drug Administration (FDA) approved Kymriah (tisagenlecleucel) for certain pediatric and…
On Apr. 11, 2016, the U.S. Food and Drug Administration (FDA) announced that it had granted accelerated approval…

On Dec. 1, 2013, researchers at the Princess Margaret Hospital in Toronto, led by Dr. John Dick, announced…

On Feb. 22, 2011, Foster City, California-based Gilead Sciences and Calistoga Pharmaceuticals announced Gilead’s acquisition of Calistoga for…
On Nov. 5, 2008, Siteman Cancer Center and The Genome Center researchers Timothy J. Ley, MD, Elaine Mardis,…
On Jan. 23, 2007, results from a large phase III clinical trial show that adult patients with previously…
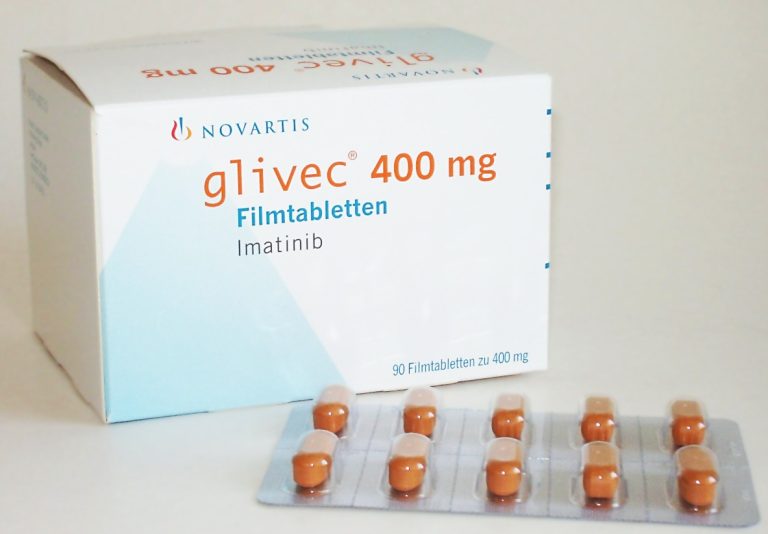
On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) granted Glivec® (imatinib) has received US regulatory…