UN to vaccinate Gaza’s children against polio during pauses in fighting
On Aug. 30, 2024, the United Nations (UN) announced that polio vaccination will begin for some 640,000 children…
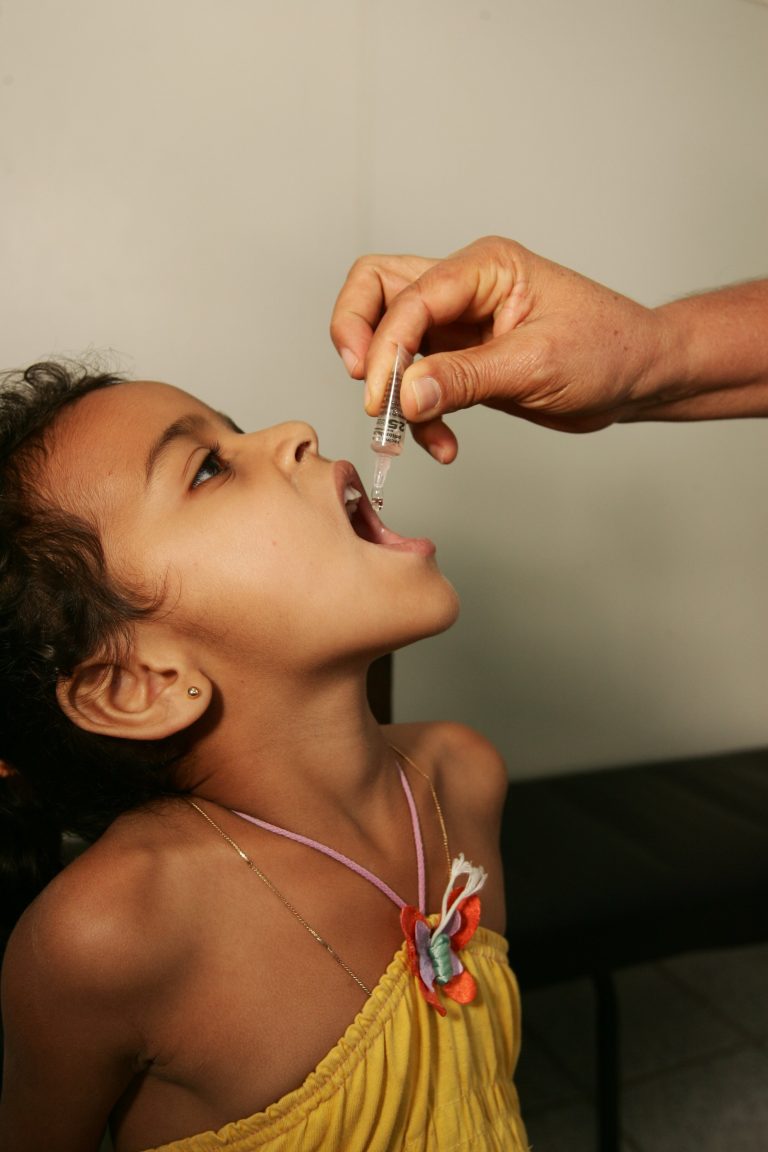
On Aug. 30, 2024, the United Nations (UN) announced that polio vaccination will begin for some 640,000 children…

On Nov. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Oct. 6, 2022, Merck announced data from two real-world evidence studies evaluating LAGEVRIOル (molnupiravir), an investigational oral…

On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…

On Mar. 29, 2022, Moderna announced that it had received approval from the U.S. Food and Drug Administration…

On Mar. 15, 2022, Pfizer and BioNTech announced the companies had submitted an application to the U.S. Food…

On Feb. 22, 2022, Moderna announced a distribution service agreement with Adium Pharma, a leading private Latin American…

On Jan. 28, 2022, Novavax and Israel’s Ministry of Health announced an agreement for the purchase of NVX-CoV2373,…

On Dec. 5, 2021, Johnson & Johnson announced preliminary results from an independent study, including a subset of…

On Aug. 3, 2021, CytoDyn announced that Brazil’s regulatory authority ANVISA (Agência Nacional de Vigilância Sanitária) has approved…

On Jun. 16, 2021, Pfizer and The Academic Research Organization (ARO) from the Hospital Israelita Albert Einstein announced…

On Jun. 7, 2021, Moderna announced a new agreement to commercialize its COVID-19 Vaccine across Central Eastern Europe…

On May 5, 2021, CytoDyn announced an agreement to partner with the Albert Einstein Israelite Hospital (AEIH) in…

On Apr. 29, 2021, scientists from several hospitals and research centers reported what happens in individual cells of…

On Apr. 28, 2021, XPhyto announce that it has delivered 2,000 of its rapid 25-minute PCR tests (モCovid-ID…

On Apr. 20, 2021, Moderna announced a new supply agreement with Israel for 2022 whereby Israel also retained…

On Mar. 11, 2021, the Israel Ministry of Health (MOH), Pfizer and BioNTech announced real-world evidence demonstrating dramatically…

On Jan. 15, 2021, VBI Vaccines announced that results from a Phase 4 study of VBIメs prophylactic 3-antigen…

On Jan. 4, 2021, Moderna announced that Israel’s Ministry of Health (MOH) had given authorization to import the…

On Dec. 1, 2020, VBI Vaccines announced the submission of a Biologics License Application (BLA) to the FDA…

On Nov. 18, 2020, Todos Medical announced positive clinical proof of concept data from its lab-based rapid SARS-CoV-2…

On Sept. 25, 2020, Todos Medical announced that it had entered into an implementation and equipment financing partnership…

On Sept. 3, 2020, Oregon Health & Science University (OHSU) announced that for the first time, early research…
On Sept. 1, 2020, Sanofi announced that the global Phase 3 trial investigating intravenously administered Kevzara (sarilumab) at…

On Jul. 8, 2020, Constant Therapeutics reported that its peptide drug TXA127 was tested in a series of…
On Jun. 24, 2020, RedHill Biopharma announced that results from the treatment of the first severe COVID-19 patients…

On Apr. 27, 2020, RedHill Biopharma provided an additional update on the compassionate use program with its investigational…

On Apr. 13, 2020, RedHill Biopharma announced the first two patients were treated with opaganib at a leading…

On Apr. 7, 2020, Pluristem Therapeutics announced preliminary data from its compassionate use program, treating seven patients suffering…