Controversial COVID study that promoted unproven hydroxychloroquine treatment retracted after four-year saga
On Dec. 18, 2024, a study that stoked enthusiasm for the now-disproven idea that the cheap malaria drug…

On Dec. 18, 2024, a study that stoked enthusiasm for the now-disproven idea that the cheap malaria drug…
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Oct. 15, 2020, the World Health Organization (WHO) announced Interim that results from the Solidarity Therapeutics Trial,…

On Sept. 30, 2020, researchers from the Perelman School of Medicine at the University of Pennsylvania reported results…

On Aug. 24, 2020, the combination of hydroxychloroquine and azithromycin has been linked to significant cardiovascular risks, including…
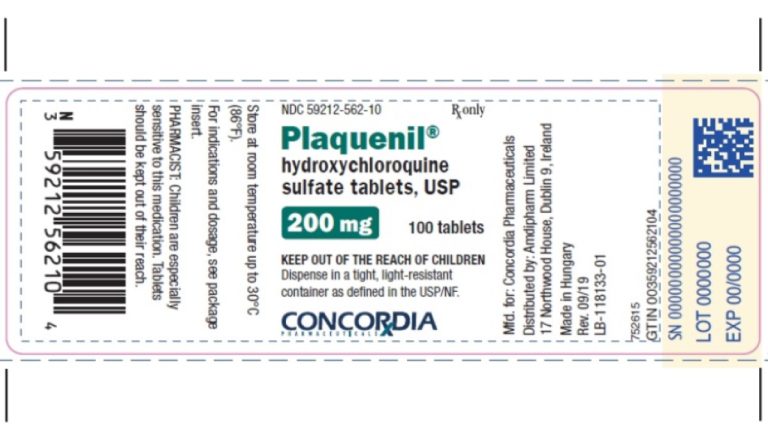
On Jul. 4, 2020, the World Health Organization (WHO) accepted the recommendation from the Solidarity Trial’s International Steering…

On Jun. 20, 2020, the National Institutes of Health (NIH) announced that a clinical trial to evaluate the…
On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) warned health care providers about a newly…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) revoked the emergency use authorization (EUA) that…

On Jun. 5, 2020, the Chief Investigators of the randomised evaluation of COVid-19 thERapY (RECOVERY) trial on hydroxychloroquine…

On May 14, 2020, the National Institute of Allergy and Infectious Diseases (NIAID) announced that a clinical trial…

On May 11, 2020, a study was published in JAMA Network that attempts to answer the question whether…

On May 7, 2020, a study of nearly 1,400 patients with moderate to severe COVID-19 disease at a…

On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine announced that they…

On Apr. 24, 2020, the U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication regarding known…

On Apr. 21, 2020, a panel of U.S. physicians, statisticians, and other experts developed treatment guidelines for coronavirus…

On Apr. 21, 2020, a study posted on medRxiv found no evidence that use of hydroxychloroquine, either with…

On Apr. 20, 2020, Novartis announced it had reached an agreement with the U.S. Food and Drug Administration…

On Apr. 13, 2020, a province wide trial was announced by Alberta Hope that will investigate the effectiveness…

On Apr. 13, 2020, the U.S. Food and Drug Administration (FDA) announced that given the increased demand for…
On Apr. 9, 2020, the first participants were enrolled in the trial at Vanderbilt University Medical Center, Nashville,…

On Apr. 9, 2020, Washington University School of Medicine in St. Louis launched a clinical trial for patients…

On Apr. 3, 2020, a trial led by the Perelman School of Medicine at the University of Pennsylvania…

On Mar. 28, 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to…

On Mar. 26, 2020, Amneal Pharma announced it had donated 1,000,000 hydroxychloroquine sulfate tablets to the Texas State…

On Mar. 20, 2020, Novartis announced its commitment to donate up to 130 million doses of generic hydroxychloroquine…

On Mar. 20, 2020, Amneal Pharma announced that it was responding to the national COVID-19 health emergency by…

On Mar. 19, 2020, Mylan announced it had restarted production of hydroxychloroquine sulfate tablets at its West Virginia…
On Mar. 19, 2020, Teva announced the immediate donation of more than 6 million doses of hydroxychloroquine sulfate…