AAP’s 2026 immunization schedule keeps routine recommendations intact after overhaul of federal schedule Free
On Jan. 26, 2026, the American Academy of Pediatrics (AAP) released its 2026 immunization schedule and continues to…

On Jan. 26, 2026, the American Academy of Pediatrics (AAP) released its 2026 immunization schedule and continues to…
On Oct. 1, 2025, Kaiser Permanente Washington announced results of the first study within a U.S.-based health system…

On Aug. 15, 2025, Precigen announced that the US Food and Drug Administration (FDA) has approved PAPZIMEOS™ (zopapogene imadenovec-drba)…
On Nov. 27, 2024, researchers at MUSC Hollings Cancer Center reported that cervical cancer deaths have plunged dramatically…
On Oct. 4, 2024, the World Health Organization (WHO) announced that a fourth WHO-prequalified human papillomavirus (HPV) vaccine…
On Aug. 5, 2024, Novartis and Viatris were named a federal lawsuit in Maryland by the family of…

On May 14, 2024, Roche announced the U.S. FDA approval of its human papillomavirus (HPV) self-collection solution, one…
On Jan. 25, 2024, an historic study out of Scotland showed the real-world impact of vaccines against the…
On Nov. 2, 2023, Abbott announced that it had received U.S. Food and Drug Administration (FDA) approval for…
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…

On Jan 5, 2022, Hologic the addition of the Aptimaᆴ SARS-CoV-2 assay to its Global Access Initiative, a…

On Oct. 13, 2021, the World Health Organization (WHO) honoured the late Henrietta Lacks with a WHO Director-General’s…

On Nov. 13, 2020, Albert Einstein College of Medicine announced that it had received a five-year, $4.9 million…
On Jul. 22, 2020, BD (Becton, Dickinson) announced that it had received approval for a pre-market approval (PMA)…

On Jul. 18, 2020, a Health Trendsル study from researchers at Quest Diagnostics and the University of Pittsburgh…
On Jul. 8, 2020, a Health Trendsル study from researchers at Quest Diagnostics and the University of Pittsburgh…

On Jun. 12, 2020, the FDA announced it had approved an expanded indication for Merck’s GARDASIL9 for the…

On Jun. 4, 2020, vaccine manufacturers MSD, GSK, Innovax, Serum Institute of India and Walvax pledged to ramp…

On Jun. 4, 2020, vaccine manufacturers MSD, GSK, Innovax, Serum Institute of India (SII) and Walvax pledged to…

On Aug. 16, 2019, the Centers for Disease Control and Prevention (CDC) published updated Immunization Practices Advisory Committee…

On Oct. 25, 2018, the American Dental Association adopted a policy to support the use and administration of…
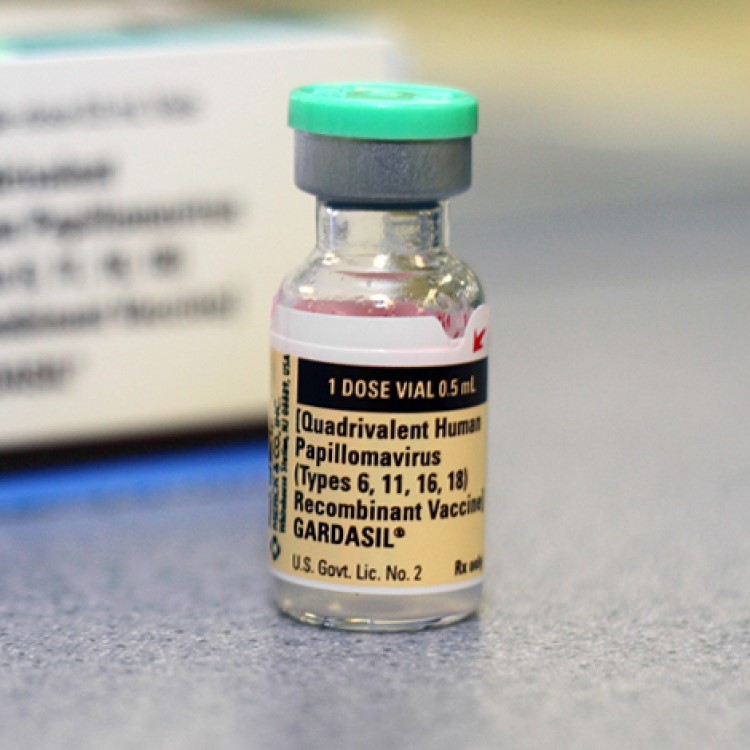
On Oct. 5, 2018, the FDA announced it had approved a supplemental application for Gardasil 9 (Human Papillomavirus…

On Aug. 24, 2018, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it passed a $2…

On Aug. 2, 2018, Emory University and GeoVax Labs partnered to develop a vaccine for HPV, with an…

On Jan. 26, 2018, the U.S. Centers for Disease Control and Prevention’ (CDC) Immunization Practices Advisory Committee (ACIP)…
On Dec. 16, 2016, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Dec. 14, 2015, the U.S. Food and Drug Administration (FDA) announced it had expanded Merck’s Gardasil 9…

On Mar. 27, 2015, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Dec. 11, 2014, Merck announced that the FDA had approved GARDASILᆴ9 (Human Papillomavirus 9-valent Vaccine, Recombinant), Merckメs…

On Dec. 16, 2013, the Centers for Disease Control and Prevention (CDC) was informed by Merck that the…