FDA approved four vaccines against the 2009 H1N1 influenza virus
On Sept. 15, 2009, the U.S. Food and Drug Administration (FDA) approved four Influenza A (H1N1) 2009 Monovalent…
On Sept. 15, 2009, the U.S. Food and Drug Administration (FDA) approved four Influenza A (H1N1) 2009 Monovalent…

Apr. 1, 2008, a team of researchers from Stanford’s School of Medicine announced they had developed a new…

On Sept. 27, 2007, the FDA Amendments Act of 2007 amended the Federal Food, Drug, and Cosmetic Act…
On Jan. 23, 2007, results from a large phase III clinical trial show that adult patients with previously…
In 2007, Dr. Jordan Tang, led a team of scientists at Oklahoma Medical Research Foundation (OMRF) that identified…
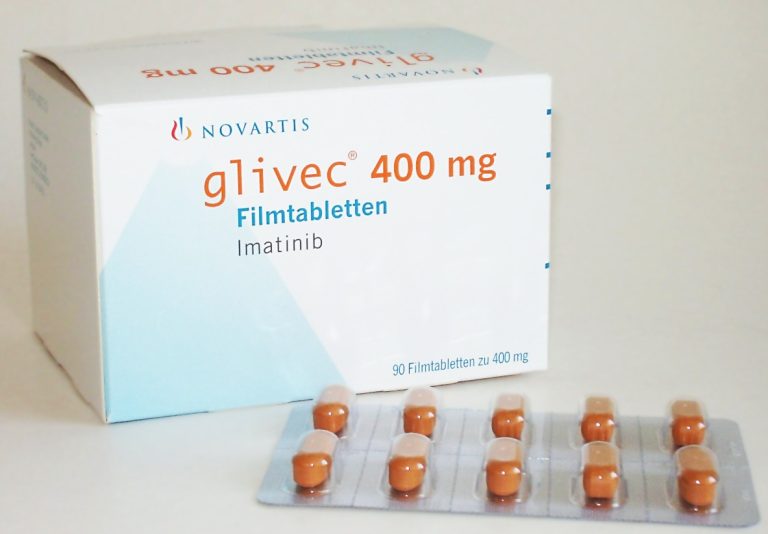
On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) granted Glivec® (imatinib) has received US regulatory…

On Oct. 5, 2006, the Foundation for the National Institutes of Health, NIH, FDA, and the Pharmaceutical Research…

On May 5, 2006, the Cancer Research Initiative was established in South Carolina, between MUSC, University of South…

In 2006, the National Cancer Institute (NCI) launched the TAILORx trial to determine whether gene expression patterns in…

On Apr. 27, 2005, results from two large National Cancer Institute sponsored randomized clinical trials showed that patients…

On May 27, 2004, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had awarded contracts…

In 2004, the Sarah Cannon Research Institute was formed to bring together community-based physicians, researchers and clinicians to…

On Oct. 9, 2003, a Canadian-led international clinical trial found that post-menopausal survivors of early-stage breast cancer who…

On Mar. 5, 2003, taking daily aspirin for as little as 3 years was shown to reduce the…
In 2003, China granted the world’s first regulatory approval of a gene therapy product, Gendicine (Shenzhen SiBiono GenTech),…
In 2001, the drug imatinib mesylate (Gleevec) was shown in a clinical trial to be effective against chronic…

On Dec. 16, 2002, researchers at Stanford University Medical Center announced they were continuing a multi-year clinical trial…

In 2001, the Stephenson Cancer Center was established. Located on the University of Oklahoma (OU) Health Sciences Center…

On Jun. 7, 2000, President Clinton issued an Executive Memorandum that directed the Medicare program to revise its…

On Apr. 6, 2000, a $60 million program was announced to address the unequal burden of cancer within…

On Oct. 20, 1997, the Food and Drug Administration (FDA) licensed a new rabies vaccine for both pre-exposure…

In 1997, the Penn Medicine’s Cancer Center was renamed the Abramson Cancer Center of the University of Pennsylvania…

In 1993, Bristol-Myers Squibb launched TAXOL (paclitaxel). The company invested hundreds of millions of dollars to supply TAXOL…
On Sept. 14, 1990, W. French Anderson and his colleagues at the National Institutes of Health (NIH) performed…

On Dec. 29, 1989, the U.S. Food and Drug Administration (FDA) approved Oculinum’s (onabotulinumtoxinA) for treatment of strabismus…

In 1989, H. Stewart Parker became CEO of Targeted Genetics, an Immunex Corp. spin-off in Seattle. which specialized…

In 1986, Dr. Marilyn Hughes Gaston while deputy branch chief of the Sickle Cell Disease Branch at the…

In Dec. 1985, the first clinical tests were held at the University of Washington of erythropoietin (EPO), the…

On Jul. 16, 1983, the National Cancer Institute launched the Community Clinical Oncology Program (CCOP) to provide a…

In 1983, the National Cancer Institute’s Division of Resources, Centers and Community Activities was renamed the Division of…