Most U.S. adults have hearts older than their actual age
On Jul. 30, 2025, a study led by Northwestern Medicine shows that most U.S. adults have a “heart…

On Jul. 30, 2025, a study led by Northwestern Medicine shows that most U.S. adults have a “heart…
On Jul. 23, 2025, research at Tufts and elsewhere has revealed the eight essential nutrients that make up…

On Jul. 16, 2025, Duke Health has pioneered a world’s-first technique that could expand by up to 20%…
On Jul. 2, 2025, researchers from the University of California, Los Angeles (UCLA) announced they have successfully grown…

On Jun. 17, 2025, Eli Lilly and Verve Therapeutics, a Boston-based clinical-stage company developing genetic medicines for cardiovascular…

On May 7, 2025, researchers at the University College London (UCL) and King’s College London announced that an…

On Apr. 3, 2025, a Stanford-led study has found that the gene CXCL12 is connected to the posterior…

On Apr. 2, 2025, Northwestern University engineers announced they have developed a pacemaker so tiny that it can…

On Mar. 25, 2025, Merck announced it has signed a licensing agreement for a heart disease drug with…

On Mar. 25, 2025, research from the American College of Cardiology reported that tiny fragments of plastic have…

On Mar. 20, 2025, Alnylam Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approval of the supplemental…

On Mar. 20, 2025, Rice University, electrical and computer engineer Kaiyuan Yang unveiled a first-of-its-kind authentication protocol for…

On Mar. 11, 2025, researchers at St Vincent’s Hospital Sydney announced the first implant of a BiVACOR Total…

On Mar. 3, 2025, the Institute for Health Metrics and Evaluation reported that without urgent policy reform and…
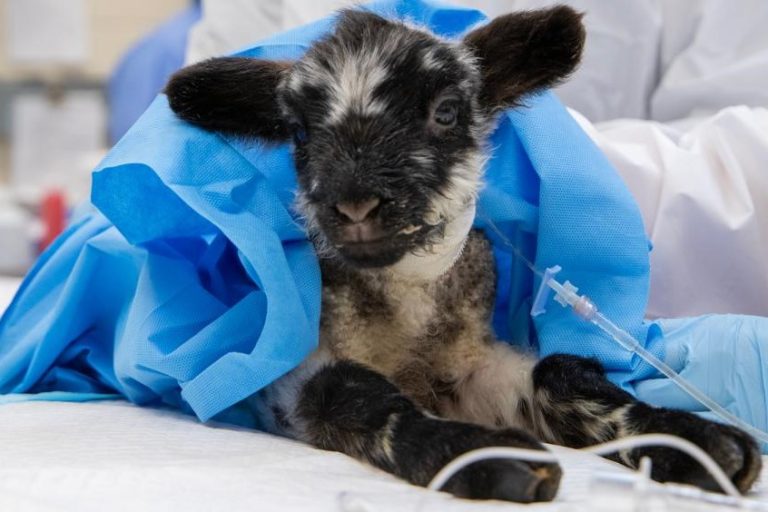
On Mar. 3, 2025, researchers from Oregon State University (OSU) announced the world’s first procedure successfully fixes lamb’s…

On Mar. 3, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved TNKase® (tenecteplase),…
On Jan. 29, 2025, researchers from the University Medical Center Göttingen announced they have shown that patches of…

On Jan. 28, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has approved Ozempic® to…

On Dec. 18, 2024, BiVACOR, a clinical-stage medical device company, announced the successful completion of the first phase…

On Dec. 16, 2024, a study published in BMC Cardiovascular Disorders shows that pediatric and young adult COVID-19…

On Dec. 5, 2024, researchers Dr Sonia Shah and Dr Clara Jiang from the University of Queensland’s Institute…
On Nov. 20, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Oct. 9, 2024, a study led by Johns Hopkins Medicine researchers concluded that commonly used ways of…

On Jul. 25, 2024, doctors from the Texas Heart Institute (THI), BiVACOR®, Baylor St. Luke’s Medical Center and…

On Jun. 28, 2024, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Jun. 26. 2024, the World Health Organization reported that new data show that nearly one third (31%)…

On Jun. 12, 2024, scientists at Weill Cornell Medicine in New York City reported that just a few…
On Apr. 30, 2024, National Institutes of Health (NIH) researchers and collaborators announced they had discovered over 100…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…