FDA approved new therapy for rare form of blood cancers called myelodysplastic syndromes
On Oct. 24, 2023, the U.S. Food and Drug Administration approved Servier Pharmaceuticals’ Tibsovo (ivosidenib) for the treatment…

On Oct. 24, 2023, the U.S. Food and Drug Administration approved Servier Pharmaceuticals’ Tibsovo (ivosidenib) for the treatment…

On Oct. 14, 2023, Purdue University ensured that the memory of the former graduate and devoted Boilermaker football…

On Oct. 7, 2023, California became the first state to ban four chemicals used in well-known candies and…

On Sept. 29, 2023, the U.S. Food and Drug Administration (FDA) granted Invitae de novo marketing authorization for…
On Sept. 27, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that new diagnoses of…

On Sept. 11, 2023, researchers at the National Institutes of Health (NIH) reported that living in an area…
On Sept. 10, 2023, the White House announced $240 million in additional investment through the Advanced Research Projects…

On Sept. 6, 2023, the Markey Cancer Center (MCC) became Kentucky’s first and only National Cancer Institute (NCI)-Designated…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…

On Aug. 14, 2023, the National Institute of Health announced that it had released a comprehensive dataset that…
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…

On Jun. 26, 2023, Jaguar Health announced that the Take C.H.A.R.G.E. (Canine Health And ReGistry Exchange) had joined…
On May 3, 2023, National Institutes of Health (NIH) scientists announced that they had uncovered new details on…

On Apr. 17, 2023, the U.S. FDA approved Gamida Cell’s Omisirge (omidubicel-onlv), a substantially modified allogeneic (donor) cord…

On Apr. 3, 2023, the U.S. Department of Health and Human Services (HHS) released a National Cancer Plan,…

On Mar. 27. 2023, Thermo Fisher Scientific and the University of California, San Francisco, announced they will accelerate…

On Mar. 23, 2023, the Fred Hutchinson Cancer Center (FHCRC) announced a new building in South Lake Union…

On Mar. 13, 2023, Pfizer announced an agreement to acquire Seagen, a global biotechnology company that discovers, develops…
On Mar. 7, 2023, researchers at the National Institutes of Health reported the benefits of screening adult patients…
On Feb. 21, 2023, Gilead Sciences announced positive data from three retrospective real-world studies which demonstrated that initiation…

On Feb. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a major step forward…

On Jan. 13, 2023, a team of researchers including faculty at the Translational Genomics Research Institute (TGen), part…

On Jan. 6, 2023, the Purdue Center for Cancer Research announced it was changing its name to the…
On Jan. 5, 2023, BioNTech announced that the Company had signed a Memorandum of Understanding with the Government…

On Dec. 16, 2022, the U.S. Food and Drug Administration approved Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firadenovec-vncg), a non-replicating…
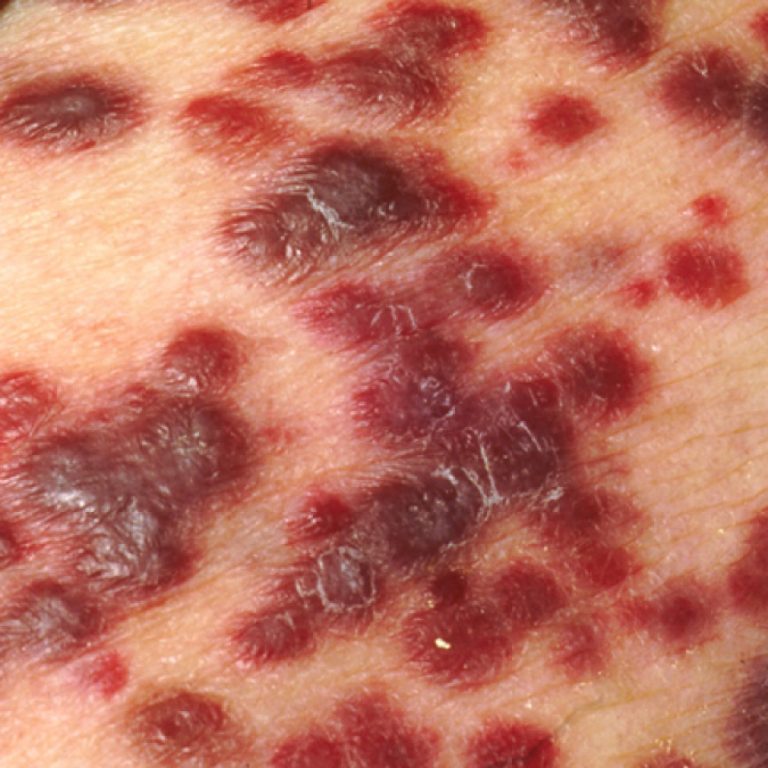
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…
On Dec. 8, 2022, the Department of Veterans Affairs embarked on a study to determine the most effective…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Oct. 26, 2022, the Barbara Ann Karmanos Cancer Institute announced it had completed an over 50,000-square-foot expansion…