Virginia Health Officials Confirm First 2025 Measles Case
On Apr. 19, 2025, the Virginia Department of Health (VDH) reported the state’s first measles case of the…

On Apr. 19, 2025, the Virginia Department of Health (VDH) reported the state’s first measles case of the…
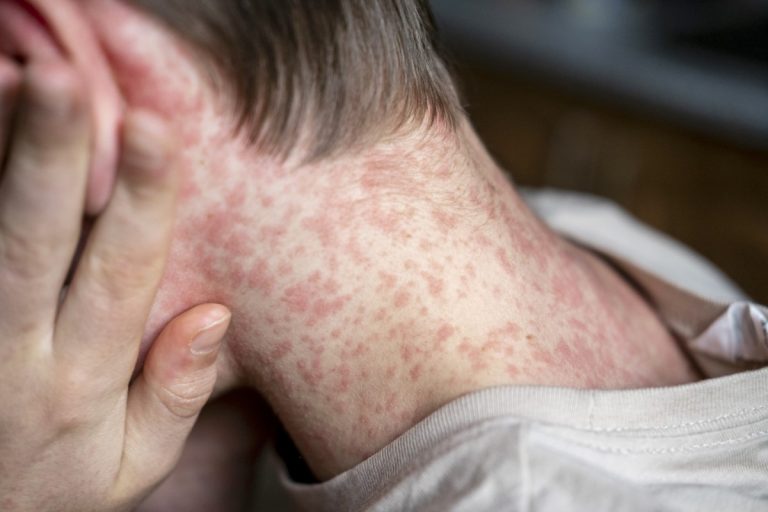
On Apr. 18, 2025, the Texas Department of State Health Services (DSHS) reported that 597 cases of measles…
On Apr. 17, 2025, a study was published that shows the United States spent roughly US$12 billion on…
On Apr. 17, 2025, the Department of Public Health and Human Services (DPHHS) and the Gallatin City-County Health…

On Apr. 17, 2025, The Tennessee Department of Health confirmed two additional confirmed cases of measles in middle…

On Apr. 16, 2025, A federal panel of medical experts has recommended an expansion of RSV vaccinations for…
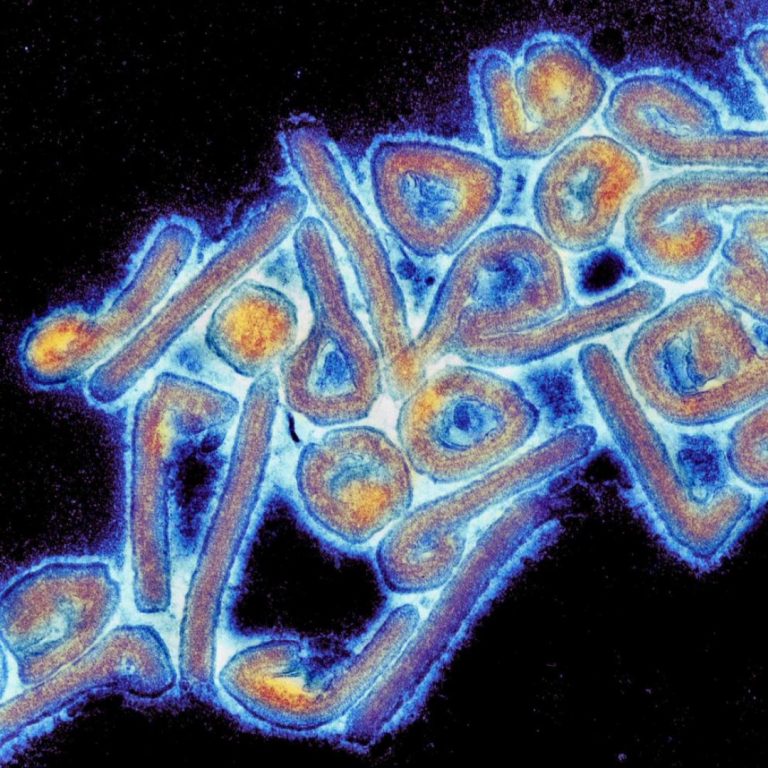
On Apr. 16, 2025, The Sabin Vaccine Institute announces the launch of a multi-site Phase 2 clinical trial…
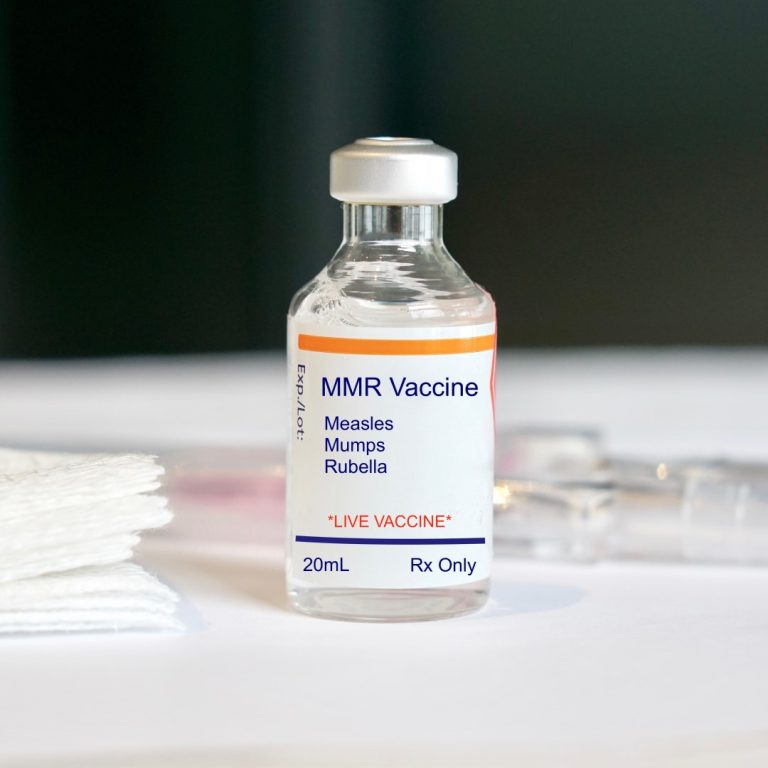
On Apr. 15, 2025, the New Mexico Department of Health (NMDOH) reports an unvaccinated child in Doña Ana…

On Apr. 15, 2025, Moderna announced that its manufacturing facility in Harwell, Oxfordshire, the Moderna Innovation and Technology…

On Apr. 15, 2025, Novavax announced preliminary results from the SHIELD-Utah study (Study of Healthcare Workers and First…

On Apr. 11, 2025, Africa’s top public health institution announced it is planning to tap more funds from…
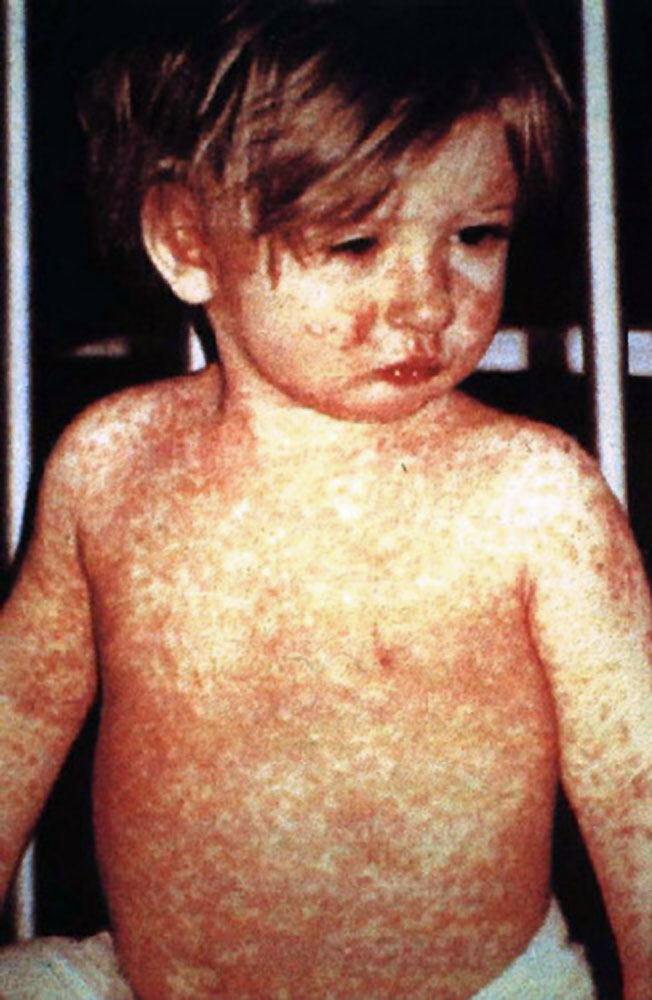
On Apr. 11, 2025, the Texas Department of State Health Services (Texas DSHS) reported that 541 cases of…
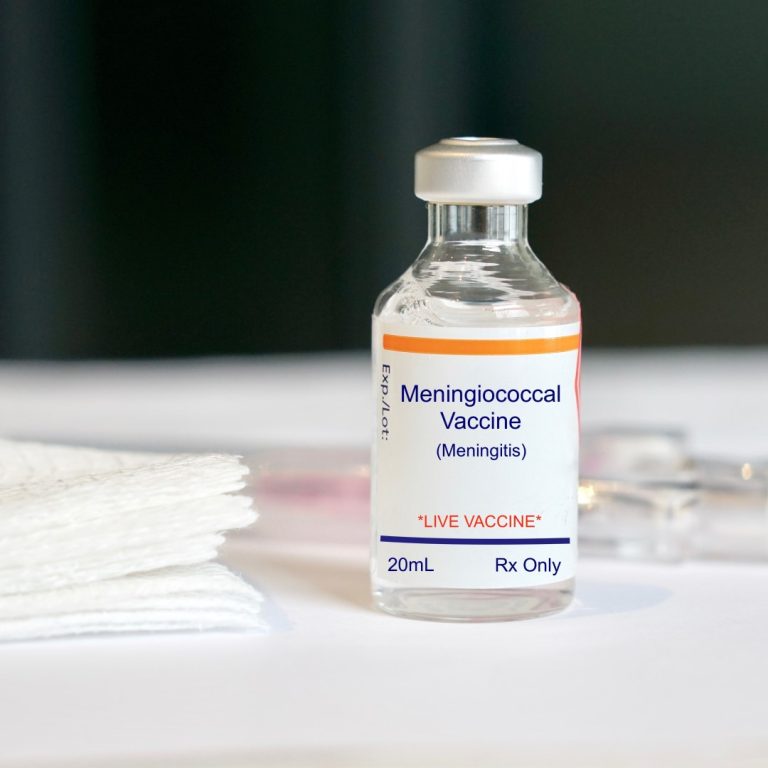
On Apr. 10, 2025, the World Health Organization (WHO) published its first-ever global guidelines for meningitis diagnosis, treatment…

On Apr. 8, 2025, the Hawaiʻi Department of Health (DOH) State Laboratories Division confirmed a case of measles…

On Apr. 7, 2025, The Indiana Department of Health (IDOH) reported the first laboratory confirmed case of measles…

On Apr. 7, 2025, the Colorado Department of Public Health and Environment issued a Health Alert Network (HAN)…

On Apr. 6, 2025, the Texas Department of State Health Services has reported the second measles death of…

On Apr. 4, 2025, a 3-year-old girl from the western state of Durango is Mexico’s first confirmed human…

On Apr. 4, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Apr. 4, 2025, the World Health Organization (WHO) announced that over the past two days, it convened…

On Apr. 3, 2025, a team from Blavatnik Institute at Harvard Medical School reported research that shows how…

On Apr. 2, 2025, the Oklahoma Children’s Hospital (OU Health) announced it had taken swift action to address…

On Apr. 2, 2025, the Colorado Department of Public Health and Environment issued a Health Alert Network (HAN)…

On Apr. 1, 2025, the Tennessee Department of Health confirmed three additional cases of measles in Middle Tennessee…

On Apr. 1, 2025, layoff notices began arriving for thousands of employees of the sprawling U.S. Department of…
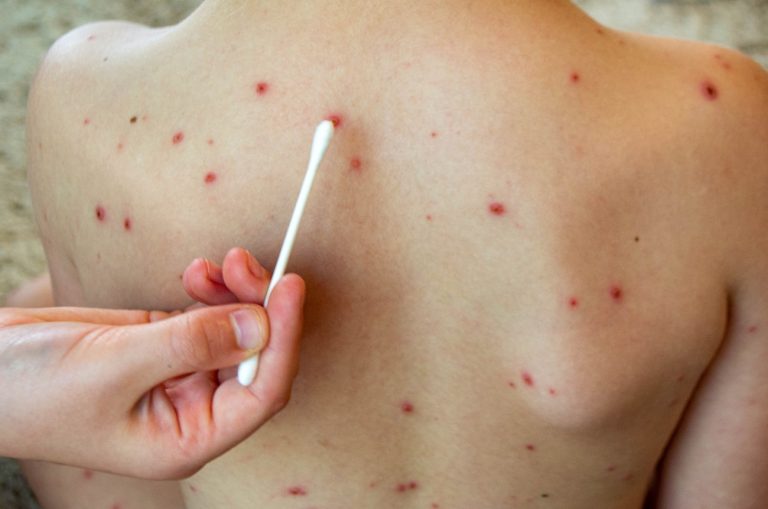
On Mar. 31, 2025, the Colorado Department of Public Health and Environment announced an unvaccinated adult in Colorado…

On Mar. 28, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Mar. 27, 2025, the Louisiana Office of the Surgeon General reported on Facebook that two infants had…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…

On Mar. 26, 2025, the Kansas Department of Health and Environment (KDHE) reported 23 positive cases of measles…