Global study to assess effects of COVID-19 in pregnancy on mothers and babies was launched
On Apr. 24, 2020, researchers at the University of Oxford announced the start of a large, international study…

On Apr. 24, 2020, researchers at the University of Oxford announced the start of a large, international study…

On Apr. 24, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) awarded $749,999 to…

On Apr. 24, 2020, the W. M. Keck Foundation announced a $4 million gift that will help scientists…

On Apr. 24, 2020, BioSig Technologies announced that subsidiary ViralClear had submitted an Investigational New Drug (IND) application…

On Apr. 24, 2020, Oregon Health & Science University (OHSU) announced that a research team led by Albert…

On Apr. 23, 2020, Emergent BioSolutions announced an agreement whereby Emergent will deploy its contract development and manufacturing…

On Apr. 23, 2020, CHF Solutions announced that it had shipped the first commercial orders of its next…

On Apr. 23, 2020, the Mayo Clinic, Minnesota Department of Health and University of Minnesota announced a breakthrough…
On Apr. 23, 2020, Western New York healthcare and medical research leaders joined forces in an effort to…

On Apr. 23, 2020, University of Oxford researchers announced they had begun testing a COVID-19 vaccine in human…

On Apr. 23, 2020, BioSig Technologies announced that an article titled, ‘The IMPDH inhibitor merimepodib has similar antiviral…

On Apr. 22, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy),…

On Apr. 22, 2020, Tyson Fresh Meats, the beef and pork subsidiary of Tyson Food, planned to indefinitely…
On Apr. 22, 2020, researchers at Massachusetts Institute of Technology (MIT); the Ragon Institute of MGH, and Harvard…

On Apr. 22, 2020, Cocrystal Pharma announced it has expanded its previously announced license agreement with Kansas State…

On Apr. 21, 2020, a panel of U.S. physicians, statisticians, and other experts developed treatment guidelines for coronavirus…
On Apr. 21, 2020, the Janssen Pharmaceutical Companies of Johnson & Johnson announced U.S. Food and Drug Administration…

On Apr. 21, 2020, a study posted on medRxiv found no evidence that use of hydroxychloroquine, either with…
On Apr. 21, 2020, BioSig Technologies announced that subsidiary ViralClear Pharmaceuticals, had submitted an application for Vicromax(tm) (merimepodib,…

On Apr. 21, 2020, Tyson Fresh Meats resumed limited operations at its pork plant in Columbus Junction, Iowa,…

On Apr. 21, 2020, Rutgers University announced it has launched the nation’s largest prospective study of health care…

On Apr. 21, 2020, Todos Medical announced that Gnomegen has received Emergency Use Authorization from the FDA for…
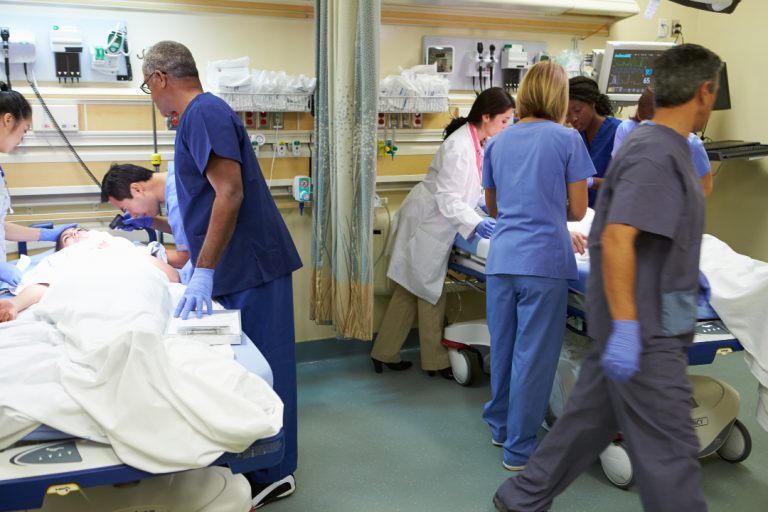
On Apr. 21, 2020, to fight the novel coronavirus pandemic, Cleveland Clinic and SAS announced they had created…

On Apr. 21, 2020, Pharnext and the University Hospital Institute Mediterranee Infection in France, announced a new joint…

On Apr. 20, 2020, the Rice University COVID-19 Research Fund Oversight and Review Committee announced it will support…

On Apr. 20, 2020, Novartis announced it had reached an agreement with the U.S. Food and Drug Administration…

On Apr. 20, 2020, RedHill Biopharma announced an agreement with the National Institute of Allergy and Infectious Diseases…

On Apr. 20, 2020, Anixa Biosciences announced it had entered into a strategic collaboration with OntoChem GmbH to…

On Apr. 20, 2020, IDEXX Labs announced the availability of the IDEXX SARS-CoV-2 (COVID-19) RealPCR Test for pets….

On Apr. 17, 2020, the NIH announced that early treatment with the experimental antiviral drug remdesivir significantly reduced…