Risk of depression and heart disease linked in women
On Dec. 5, 2024, researchers Dr Sonia Shah and Dr Clara Jiang from the University of Queensland’s Institute…
On Dec. 5, 2024, researchers Dr Sonia Shah and Dr Clara Jiang from the University of Queensland’s Institute…
On Dec. 4, 2024, The U.S. Food and Drug Administration (FDA) announced approved of AstraZeneca’s blockbuster drug Imfinzi…

On Dec. 4, 2024, artificial intelligence (AI) researchers, in an effort to automate scientific discovery announced they have…

On Dec. 4, 2024, researchers at the Centre for Genomic Regulation (CRG) in Barcelona announced they had discovered…

On Dec. 3, 2024, researchers at Swansea University in Wales announced they have developed a revolutionary bone graft…
On Dec. 3, 2024, the California Department of Public Health (CDPH) announced it had secured a broad, voluntary…
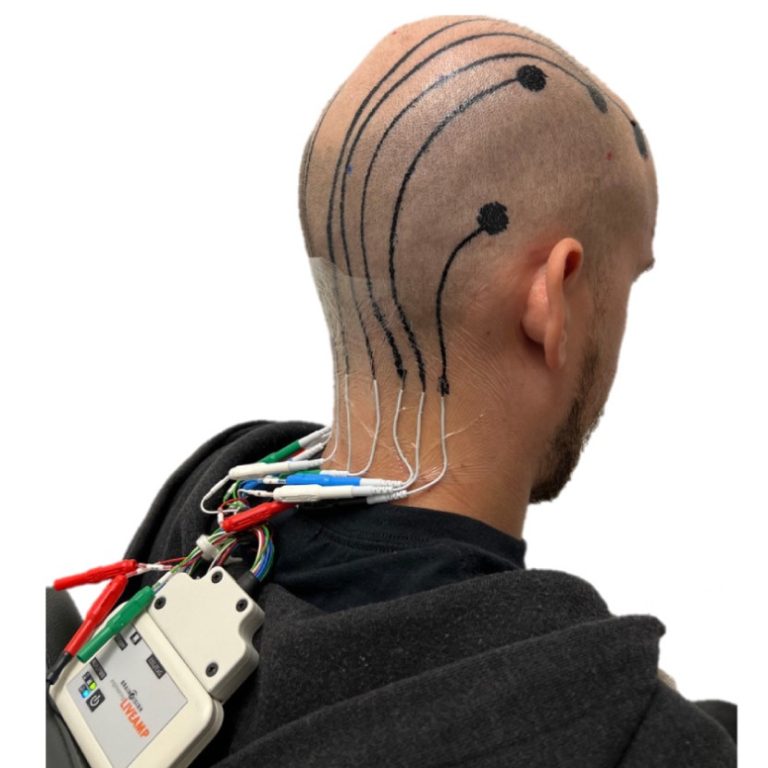
On Dec. 2, 2024, a team of researchers reported they had conducted on-scalp digital printing of custom-designed, temporary-tattoo-like…
On Dec. 2, 2024, the University of Colorado Anschutz Medical Campus announced it will receive up to $46…

On Dec. 2, 2024, engineers in the University of Southern California (USC) Alfred E. Mann Department of Biomedical…

On Nov. 28, 2024, scientists from King’s College London reported that an injection given during some asthma and…

On Nov. 27, 2024, a study led by the University College London reported that large language models, a…

On Nov. 27, 2024, the California Department of Public Health (CDPH) issued a second warning to Californians to…
On Nov. 27, 2024, researchers at MUSC Hollings Cancer Center reported that cervical cancer deaths have plunged dramatically…

On Nov. 26, 2024, scientists at Texas A&M University announced they have found that healing the gut may…
On Nov. 26, 2024, the the United Nations reported that fewer people contracted HIV last year than at…
On Nov. 25, 2024, the Texas Department of State Health Services reported that the first locally acquired case…

On Nov. 24, 2024, the California Department of Public Health (CDPH) issued a warning the public to avoid…

On Nov. 22, 2024, the Public Health Agency of Canada (PHAC) confirmed the first case of clade I…

On Nov. 21, 2024, teams at the University of Texas Southwestern Medical Center report a new way that…
On Nov. 21, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding $29.7 million to…
On Nov. 21, 2024, a study from scientists at the UCLA Health Jonsson Comprehensive Cancer Center helps explain why…

On Nov 20, 2024, researchers from the Wellcome Sanger Institute and collaborators have used cutting-edge genomic techniques to…
On Nov. 20, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov, 20, 2024, Pfizer announced that the European Commission (EC) has granted marketing authorization for HYMPAVZI™ (marstacimab)…

On Nov. 20, 2024, researchers with the global Human Cell Atlas (HCA) consortium reported significant progress in their…
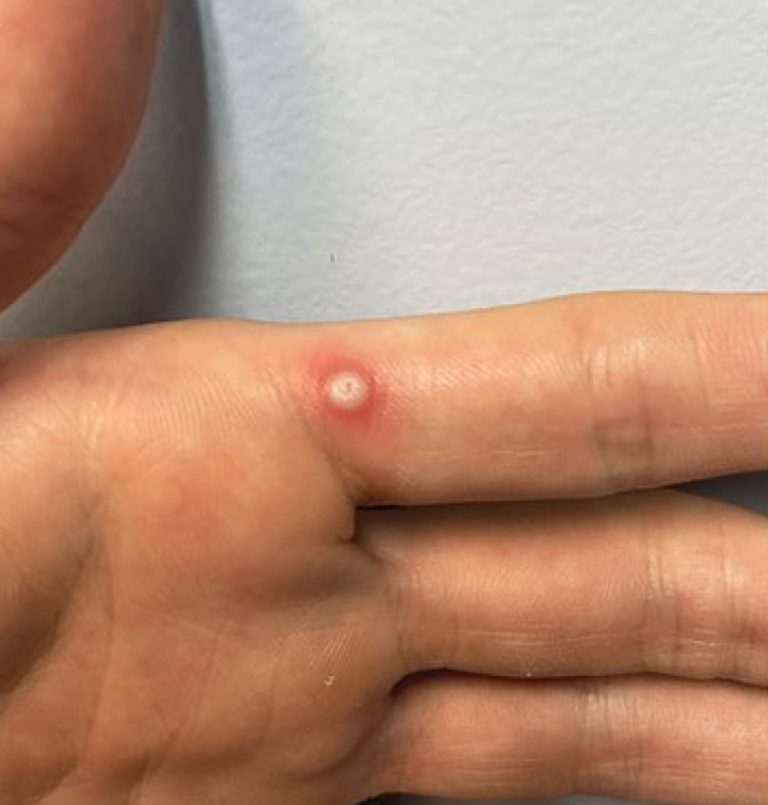
On Nov. 16, 2024, the California Department of Public Health (CDPH) announced it had identified through laboratory testing…

On Nov. 15, 2024, the White House Office of Science and Technology Policy released a report on Building…
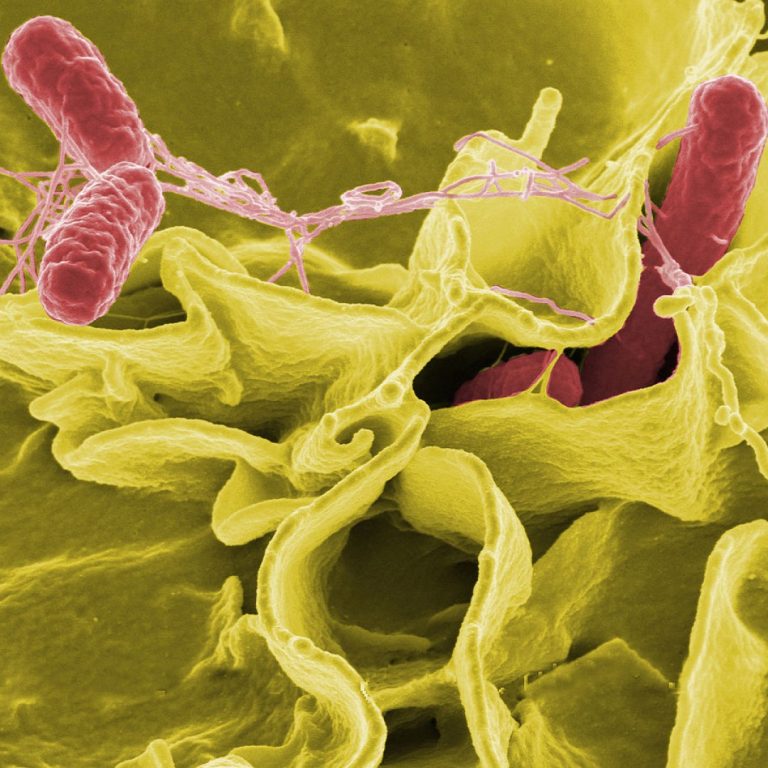
On Nov. 15, 2024, a University of California Davis Health (UC Davis Health) research team announced they had…

On Nov. 15, 2024, a team led by researchers from Sweden’s Karolinska Institutet reported study results that showed…

On Nov. 15, 2024, the AARP, the Alzheimer’s Disease Data Initiative (AD Data Initiative), and the Institute for…