CPRIT granted $18.7 million New Company Product Development award to Salarius Pharmaceuticals
On Aug. 15, 2019, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it was awarded…

On Aug. 15, 2019, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it was awarded…
On Jul. 18, 2019, Chembio Diagnostics announced a collaboration with Shire Human Genetic Therapies, a wholly owned subsidiary…

On May 30, 2019, FUJIFILM opened a Life Science Strategic Business Office in Cambridge, Massachusetts to create a…
On Jan. 23, 2019, U.S. Food and Drug Administration (FDA) announced it had approved approved the use of…

On Jan. 9, 2019, pharmaceutical company AstraZeneca announced it was laying off more than 200 employees in Boulder…

On Jan. 8, 2019, Takeda Pharmaceutical announced the completion of its acquisition of Lexington, Massachusetts-based Shire for a…

On Nov. 13, 2018, Canadian Prime Minister Justin Trudeau announced federal funding for the Institute for Advanced Medical…

On Oct. 16, 2018, Discovery Life Sciences expanded by merging with Conversant Bio, Folio Bio, and Phylogeny to…
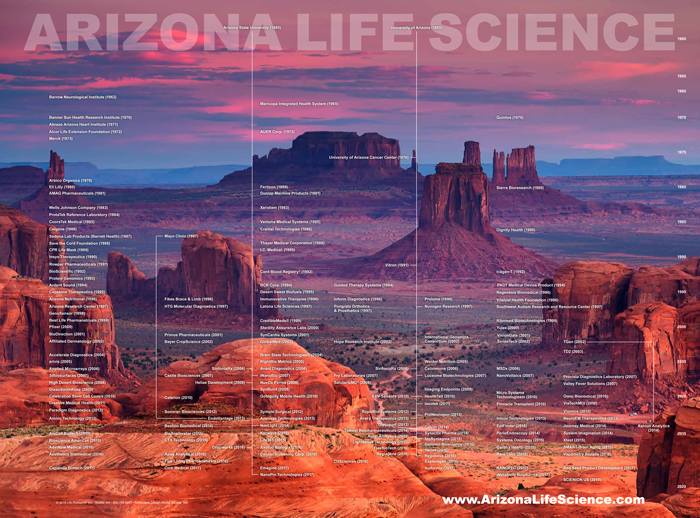
On Aug. 9, 2018, Arizona Life Science Genealogy was published illustrating life science companies and non-profit research organization…

On Jul. 6, 2018, Teva Pharmaceuticals announced it would move its U.S. headquarters to Parsippany-Troy Hills, New Jersey…
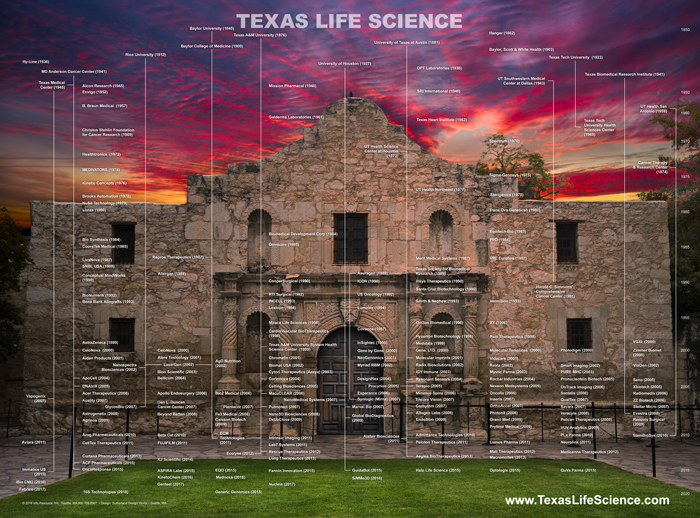
Texas Life Science Genealogy illustrates life science companies and non-profit research organization in the State of Texas by…

On Mar. 8, 2018, Sanofi acquired Waltham, Massachusetts-based Bioverativ for for $11.6 billion. Bioverativ’s extended half-life therapies, Eloctate…

On Feb. 14, 2018, Pacific Northwest National Laboratory, (PNNL) and Oregon Health & Science University OHSU announced the…
On Jan. 26, 2018, the U.S. Centers for Disease Control and Prevention’ (CDC) Immunization Practices Advisory Committee (ACIP)…

On Jan. 22, 2018, Celgene and Juno Therapeutics announced the signing of a merger agreement in which Celgene…

On Nov. 1, 2017, a study from the Earth Microbiome Project reported the most extensive snapshot ever of…

On Oct. 2, 2017, Emory University’s Center for Selective C-H Functionalization (CCHF) was awarded a $20 million grant…

On Sept. 18, 2017, Medigen Vaccine Biologics (MVC) amended an existing agreement with the National Institutes of Health…

On Aug. 1, 2017, Achieve Life Sciences announced it had merged with OncoGenex and was renamed Achieve Life…

On May 24, 2017, the National Cancer Institute (NCI) awarded $24 million to Fred Hutchinson Cancer Research Center…

In 2017, SpringWorks Therapeutics was founded in Stamford, Connecticut, as a clinical-stage biopharmaceutical company that applies a precision…
On Dec. 3, 2016, Celgene, Dana-Farber Cancer Institute and the University of Arkansas for Medical Sciences announced the…

On Nov. 21, 2016, Novartis announced it had acquired Selexys Pharmaceuticals Corp, a Oklahoma City-based company that specialized…
On Nov. 4, 2016, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP)…

On Sept. 14, 2016, Bayer and Monsanto announced a merger agreement whereby Bayer acquired Monsanto for USD $128…

On Aug. 23, 2016, University of Nebraska Medical Center (UNMC) celebrated the grand opening of the UNMC Center…

On Jul. 26, 2016, Patheon announced the closing of its initial public offering of 34,226,191 of its ordinary…

On Jun. 7, 2016, IBio and the Texas A&M Institute of Infectious Animal Diseases (TAMUS) announced they had…

On Sept. 5, 2017, business incubator TEC Edmonton announced a strategic partnership with Merck Canada to provide new…

On Mar. 14, 2016, Inovio Pharmaceuticals announced an agreement to acquire all of Bioject Medical Technologies’ assets including…