Dana-Farber Researchers Create Experimental AI-based Oncologist’s Assistant
On Jan. 20, 2026, Dana-Farber researchers announced developing an AI-based oncologist’s assistant that shows the potential for AI…

On Jan. 20, 2026, Dana-Farber researchers announced developing an AI-based oncologist’s assistant that shows the potential for AI…

On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…

On Jan. 13, 2026, collaborating with Google’s top artificial intelligence labs, a team of Yale researchers announced they…

On Jan. 13, 2026, BriaCell Therapeutics announces the durable and sustained complete resolution of a lung metastasis in…

On Jan. 13, 2026, The American Cancer Society (ACS) released Cancer Statistics, 2026, the organization’s annual report on cancer facts and trends. The findings show, for…

On Jan. 9, 2026, Novartis announced plans to build its fourth US radioligand therapy (RLT) manufacturing facility in…
On Jan. 9, 2026, scientists at the Icahn School of Medicine at Mount Sinai, in partnership with the Multiple Myeloma Research…
On Jan. 8, 2026, for the first time, researchers at the University of British Columbia have demonstrated how…

On Jan. 8, 2026, a major advance in cell biology has revealed how our cells safeguard their genetic…

On Jan. 6, 2026, a systematic analysis for the Global Burden of Disease Global study shows that lower…

On Jan. 6, 2026, a study led by University of Washington School of Medicine researchers uncovered several new…

On Dec. 18, 2025, the National Aeronautics and Space Administration (NASA) announced it has opened the International Space…

On Dec. 15, 2025, Microsoft Research announced a new AI tool developed by and published in Cell allows…

On Dec. 11, 2025, the California Institute for Regenerative Medicine (CIRM) governing board approved funding of over $160…
On Dec. 9, 2025, researchers at the UCLA Health Jonsson Comprehensive Cancer Center announced they have identified a…

On Dec. 9, 2025, officials from the Canada Economic Development for Quebec Regions, and the Minister of Industry,…
On Dec. 8, 2025, a study led by Roswell Park Comprehensive Cancer Center reveals that a patient’s race…

On Nov. 24, 2025, a new study led by UCLA investigators found that combining zanzalintinib, a targeted therapy…

On Nov. 21, 2025, Pfizer and Astellas Pharma announced that the U.S. Food and Drug Administration (FDA) has…

On Nov. 20, 2025, the US Food and Drug Administration (FDA) has approved the first cancer drug based…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…

On Nov. 19, 2025, the governing board of the Cancer Prevention and Research Institute of Texas (CPRIT) approved…

On Nov. 19, 2025, Novartis, a leading global innovative medicines company, announced plans to expand its operations in…

On Nov. 17, 2025, Johnson & Johnson announced that it has entered into a definitive agreement to acquire…

On Nov. 11, 2025, China introduced a visa that will allow young foreign researchers in science, technology, engineering…
On Oct. 29, 2025, the CWTS Leiden ranking show that Harvard University dropped to third place in the…

On Oct. 29, 2025, Eli Lilly announced a planned investment of more than $1.2 billion to expand and…
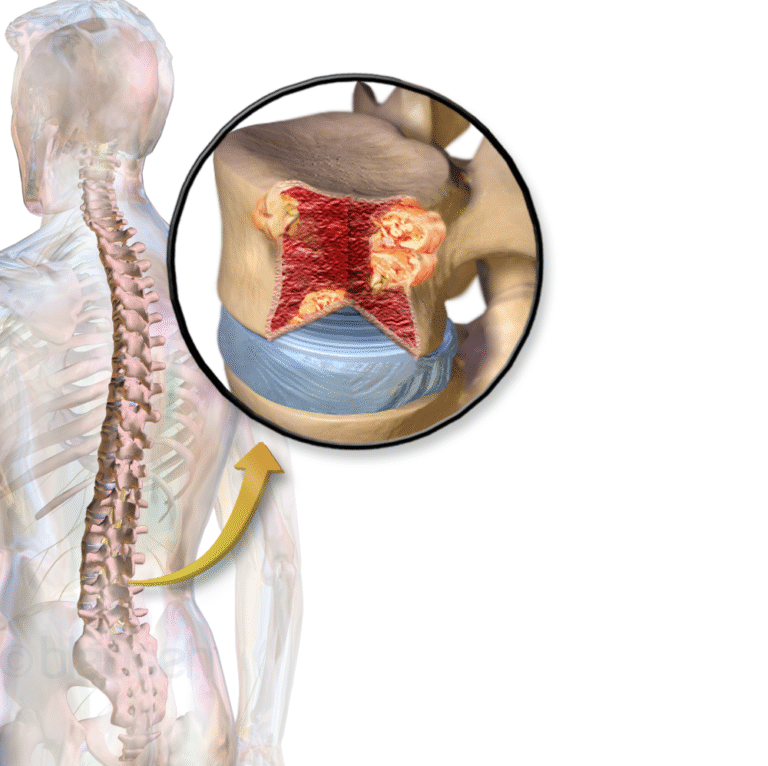
On Oct. 23, 2025, GSK announced the US Food and Drug Administration (FDA) has approved Blenrep (belantamab mafodotin-blmf)…

On Oct. 18, 2025, GRAIL announced that positive performance and safety results from its registrational PATHFINDER 2 study…

On Oct. 17, 2025, Chinese biotech Hansoh Pharma said it had signed a license agreement worth up to…