NIH announced formation of All of Us Research program
In 2015, the National Institutes of Health (NIH) announced The All of Us Research Program, a historic effort…

In 2015, the National Institutes of Health (NIH) announced The All of Us Research Program, a historic effort…

On Dec. 11, 2014, Merck announced that the FDA had approved GARDASILᆴ9 (Human Papillomavirus 9-valent Vaccine, Recombinant), Merckメs…

On Oct. 29, 2014, Pfizer announced the FDA has granted accelerated approval of TRUMENBAᆴ (meningococcal group B vaccine)…

On Oct. 13, 2014, the International Society on Thrombosis and Haemostasis launched the annual World Thrombosis Day World…

On Oct. 13, 2014, the Nebraska Medical Center, Bellevue Medical Center and UNMC Physicians – once three separate…

On Oct. 8, 2014, Ohio State University, Cardinal Health and State of Ohio Third Frontier Commission announced completion…

On Oct. 6, 2014, May-Britt Moser won a share of the 2014 Nobel Prize in Physiology or Medicine…

On Oct. 5, 2014, BD and CareFusion announced a definitive agreement whereby BD acquired CareFusion for $58.00 per…

On Sept. 23, 2014, Bristol-Myers Squibb announced plans to build a 650,000-square-foot office building on company-owned land in…

On Sept. 12, 2014, the University of Iowa announced that alumnus Franklin D. Trueblood’s lifelong interest in medical…

On Sept. 11, 2014, the National Institutes of Health established the Library of Integrated Network-based Cellular Signatures. Building…

On Sept. 3, 2014, Abbott announced it had received CE Mark (Conformite Europeenne) for its FreeStyle Libre Flash…

On Aug. 19, 2014, Baxter announced it planned to expand its state-of-the-art manufacturing facility in Opelika, to help…

On Jul. 10, 2014, an international confederation of biotechnology trade associations ratified bylaws that created the International Council…

On Jun. 28, 2014, The Burnham Institute for Medical Research announced a record $275 million anonymous to helping…

On Apr. 24, 2014 an international team of scientists announced they had deciphered the genetic code of the…
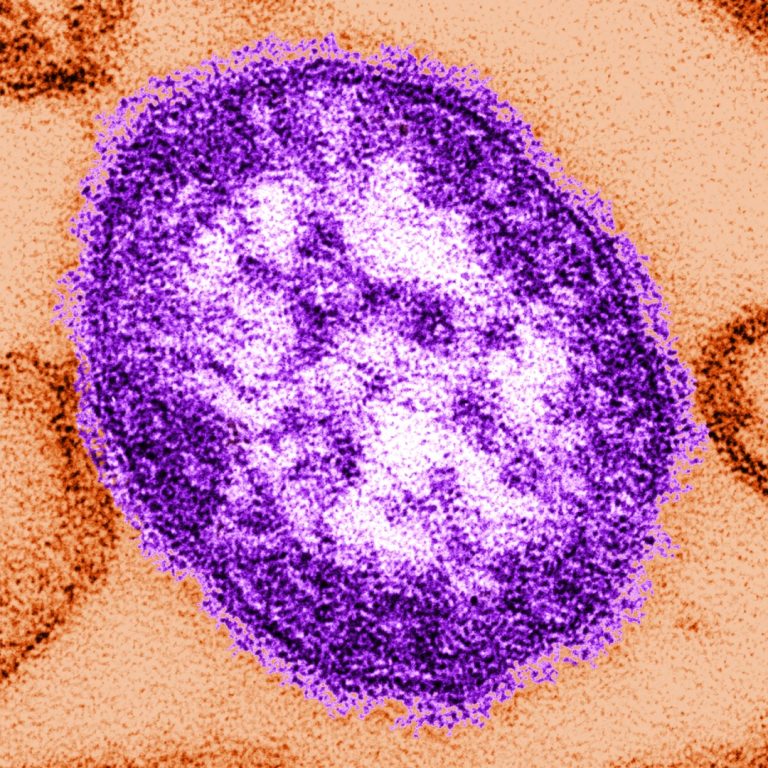
On Apr. 24, 2014, the U.S. Centers for Disease Control and Prevention (CDC) estimated that vaccinations prevent more…

On Apr. 4, 2014, Lowell Miller, a resident of Loch Lloyd, Mo., made a gift of $1.1 million…

On Apr. 1, 2014, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had expanded the approved…

On Mar. 13, 2014, the Alabama Institute of Medicine received $1 Million gift from an anonymous Birmingham resident…
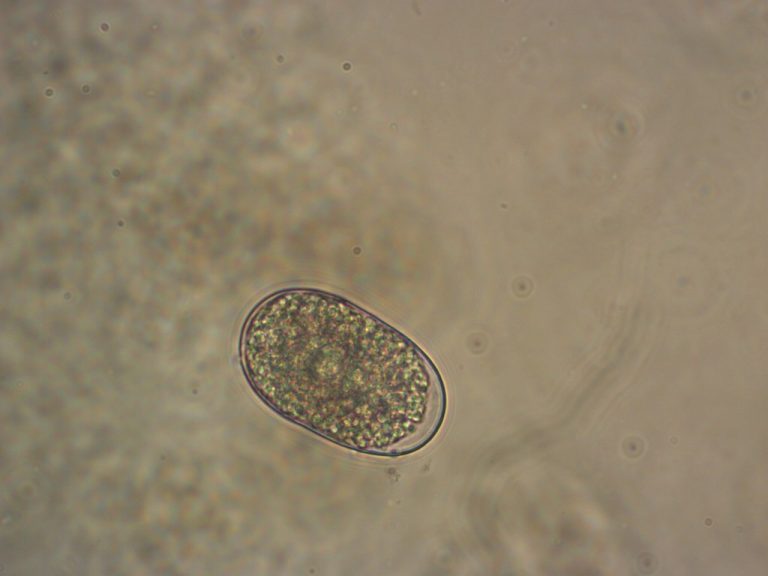
On Jan. 19, 2014, Washington University scientists announced they had decoded the genome of the hookworm, Necator americanus….
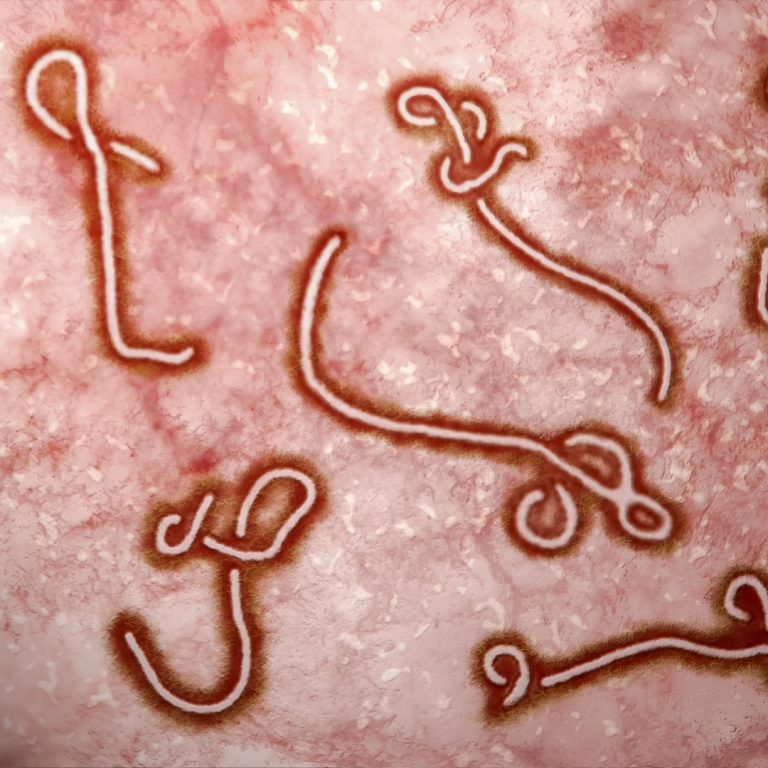
On Dec. 26, 2013, a 2-year-old boy in the remote Guinean village of Meliandou fell ill with a…
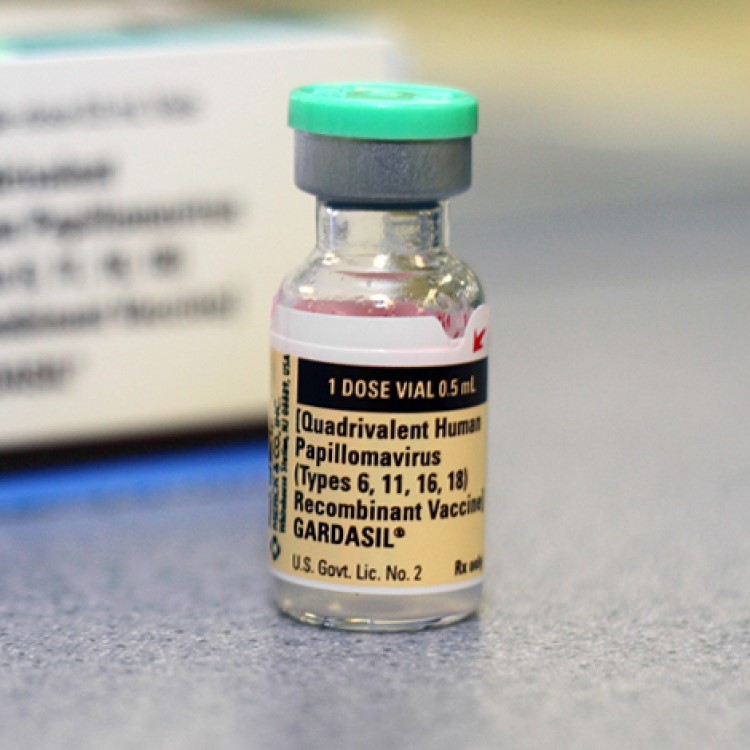
On Dec. 16, 2013, the Centers for Disease Control and Prevention (CDC) was informed by Merck that the…

On Dec. 1, 2013, researchers at the Princess Margaret Hospital in Toronto, led by Dr. John Dick, announced…

On Nov. 19, 2013, the Indiana Biosciences Research Institute (IBRI) was initially funded with $50 million provided by…

On Nov. 19, 2013, the Indiana Biosciences Research Institute announced that it had reached the first $50 million…

On Nov. 9, 2013, the Institute for Health Metrics and Evaluation (IHME) at the University of Washington launched…

On Sept. 18, 2013, it was reported a parasite that infects up to one-third of people around the…

On Sept. 17, 2013 researchers at the South Korea’s Personal Genomics Institute in Suwon reported the mapping of…

On Sept. 12, 2013, Oregon Health & Science University (OHSU) announced it had received two gifts totaling $25…