TGen study predicted changing Lyme disease habitat across the West Coast
On Mar. 16, 2021, TGen announced a study that suggested ecosystems suitable for harboring ticks that carry debilitating…

On Mar. 16, 2021, TGen announced a study that suggested ecosystems suitable for harboring ticks that carry debilitating…

On Mar. 16, 2021, U.S. military researchers announced they had found that headache, dizziness and cognitive dysfunction occur…

On Mar. 16, 2021, Roche announced the launch of the cobasᆴ SARS-CoV-2 Variant Set 1 Test to detect…

On Mar. 15, 2021, Resverlogix announced the publishing of a new article titled: モBromodomain and extraterminal protein inhibitor,…

On Mar. 15, 2021, ImmunityBio announced it had met the safety requirements for the first 12 participants in…

On Mar. 15, 2021, Altimmune announced additional preclinical data for its single dose intranasal COVID-19 vaccine candidate, AdCOVID….

On Mar. 15, 2021, Fulgent Genetics announced that the U.S. Centers for Disease Control and Prevention had awarded…

On Mar. 12, 2021, the WHO listed the COVID-19 vaccine Ad26.COV2.S, developed by Janssen (Johnson & Johnson), for…

On Mar. 12, 2021, Johnson & Johnson announced that the World Health Organization (WHO) had issued Emergency Use…

On Mar. 11, 2021, Rigel Pharma announced the completion of patient enrollment in a multi-center Phase 2 clinical…

On Mar. 11, 2021, ImmunityBio announced it was developing a novel hAd5 ACE2 Decoy therapeutic vaccine to neutralize…

On Mar. 11, 2021, Johnson & Johnson announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Mar. 11, 2021, the Israel Ministry of Health (MOH), Pfizer and BioNTech announced real-world evidence demonstrating dramatically…

On Mar. 11, 2021, the Department of Veterans Affairs (VA) announced that it had received compassionate use approval…

On Mar. 11, 2021, Abbott announced the formation of the Abbott Pandemic Defense Coalition, a first-of-its-kind global scientific…

On Mar. 10, 2021, TGen, an affiliate of City of Hope, announced the start of a scientific study…

On Mar. 10, 2021, CEPI, the Coalition for Epidemic Preparedness Innovations, and VBI Vaccines announced a partnership to…

On Mar. 10, 2021, Thermo Fisher Scientific announced more than $600 million in capital investments to expand its…
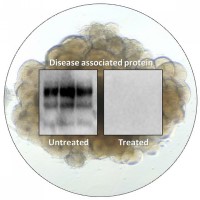
On Mar. 9, 2021, the NIH announced that approximately two years after establishing a human cerebral organoid system…

On Mar. 9, 2021, Agilent Technologies announced the launch of a real-time reverse transcription (qRT) PCR-based diagnostic kit…

On Mar. 9, 2021, NanoViricides announced that NV-CoV-2 and NV-CoV-2-R were found to be highly effective against a…

On Mar. 9, 2021, Oragenics announced it had entered into a material transfer agreement with Biodextris for the…

On Mar. 9, 2021, VBI Vaccines announced the initiation of enrollment of its Phase 1/2 clinical study of…

On Mar. 8, 2021, ImmunityBio and NantKwest announced that the first cohorts of their South Africa and U.S….

On Mar. 8, 2021, AIM ImmunoTech announced that it had dosed the first healthy subjects in its Phase…

On Mar. 5, 2021, Abivax announced tthat it was halting the miR-AGE phase 2b/3 clinical trial in high-risk…

On Mar. 5, 2021, Merck and Ridgeback Biotherapeutics, announced preliminary results from Ridgebackメs Phase 2a randomized, double-blind, placebo-controlled…

On Mar. 5, 2021, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the…
On Mar. 5, 2021, Cue Health announced it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Mar. 4, 2021, CureVac and Novartis announced an initial agreement for the manufacturing of CureVacメs COVID-19 vaccine…