Marburg outbreak in Rwanda declared over
On Dec. 20, 2024, the World Health Organization (WHO) announced that the outbreak of Marburg Virus Disease was…
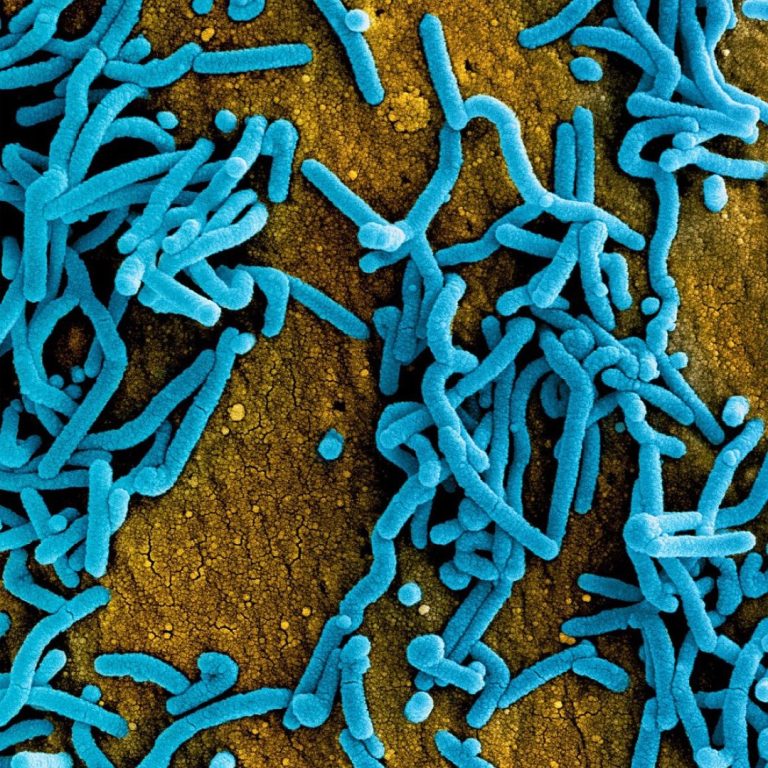
On Dec. 20, 2024, the World Health Organization (WHO) announced that the outbreak of Marburg Virus Disease was…

Dec. 19, 2024, the Los Angeles County Department of Public Health is urging residents to avoid consuming or…

On Dec. 18, 2024, a team of researchers reported results from preclinical testing of mRNA-based vaccines against Highly…

On Dec. 18, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that a patient has…
On Dec. 18, 2024, the Institute for Health Metrics and Evaluation reported that diarrhoeal diseases claim more than…

On Dec. 18, 2024, officials from the Washington Department of Fish and Wildlife (WDFW) confirmed highly pathogenic avian…

On Dec. 18, 2024, the National Academies of Sciences released a report showing the current information ecosystem makes…

On Dec. 18, 2024, Gavin Newsom, Governor of Californa, proclaimed a State of Emergency to streamline and expedite…

On Dec. 18, 2024, a study that stoked enthusiasm for the now-disproven idea that the cheap malaria drug…

On Dec. 18, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported a study with patient…

On Dec. 16, 2024, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Dec. 16, 2024, JAMA Network Open published a study demonstrating respiratory syncytial virus (RSV) vaccine effectiveness in adults…

On Dec. 16, 2024, a study published in BMC Cardiovascular Disorders shows that pediatric and young adult COVID-19…

On Dec. 13, 2024, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced $165…

On Dec. 12, 2024, University of Pittsburgh researchers announced they had identified evidence of H5N1 adaptation in domestic…
On Dec. 11, 2024, the World Health Organization (WHO) reported new data has revealed that an estimated 2.2…

On Dec. 10, 2024, the Pan American Health Organization (PAHO) reported on three transmissible diseases affecting the Region…

On Dec. 10, 2024, a research team led by Fangqiong Ling, an assistant professor at Washington University in…

On Dec. 9, 2024, the Africa Centers for Disease Control (CDC) announced that it is working closely with…

On Dec. 6, 2024, Shi Zhengli, the Chinese virologist at centre of COVID lab-leak theory, has presented evidence…

On Dec. 5, 2024, a study led by scientists at Scripps Research reveals that a single mutation in…
On Dec. 5, 2024, the World Health Organization (WHO) has granted prequalification to the molecular diagnostic test for…

On Dec. 3, 2024, Congolese officials reported that a flu-like disease that has killed dozens of people over…
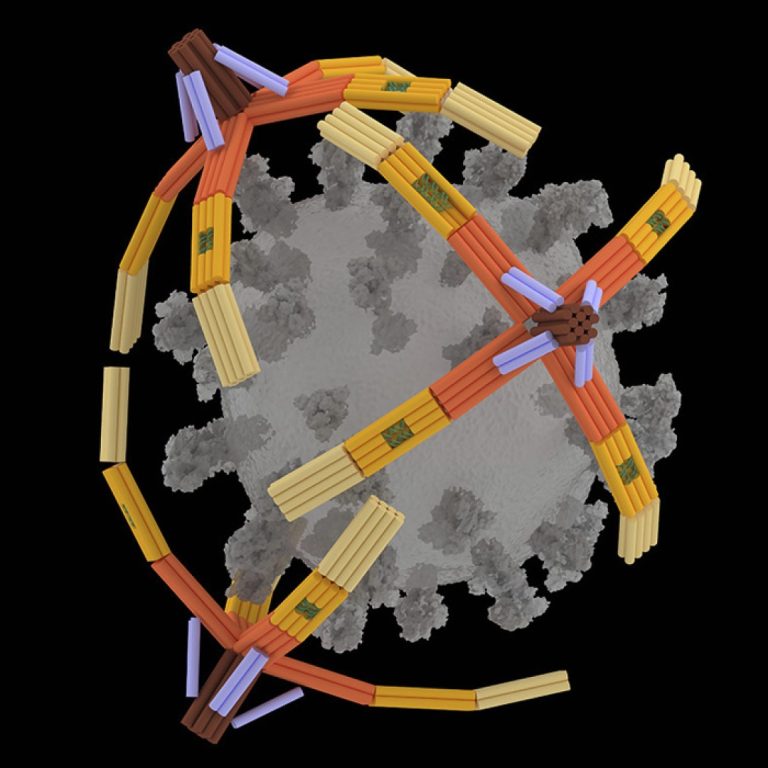
On Nov. 27, 2024, researchers at the University of Illinois Urbana-Champaign announced they had developed a nanorobotic hand…
On Nov. 27, 2024, researchers at MUSC Hollings Cancer Center reported that cervical cancer deaths have plunged dramatically…

On Nov. 26, 2024, the U.S. Department of Agriculture (USDA) announced it had temporarily paused imports of Mexican…
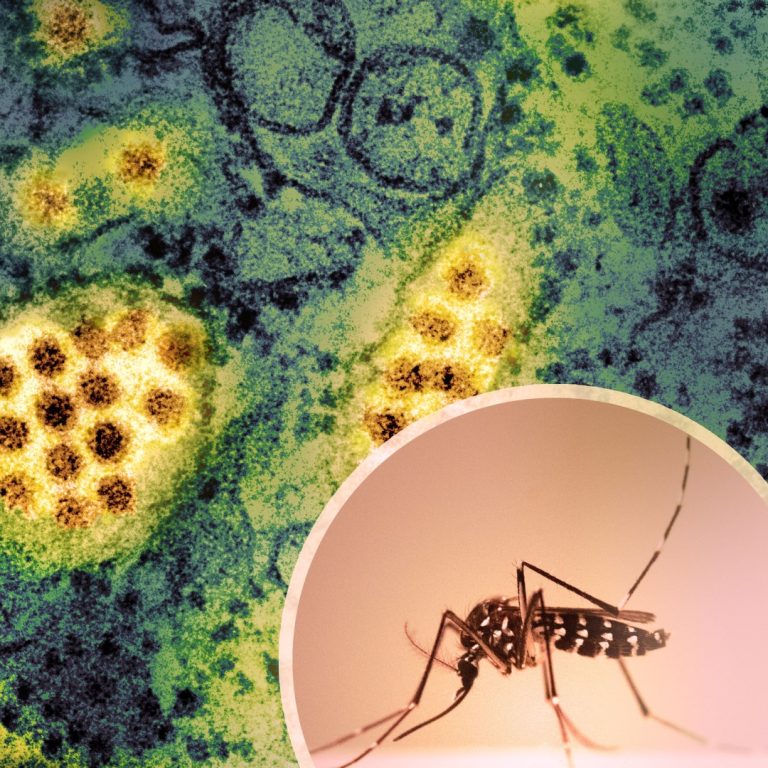
On Nov. 25, 2024, the Texas Department of State Health Services reported that the first locally acquired case…

On Nov. 22, 2024, the Public Health Agency of Canada (PHAC) confirmed the first case of clade I…

On Nov. 22, 2024, the Chief Veterinary Officer of Mexico notified the U.S. Department of Agriculture’s (USDA) Animal…

On Nov. 21, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that by the week…