FDA Modernization Efforts for Establishing a Unified Human Foods Program launched
On Oct. 1, 2024, the U.S. Food and Drug Administration (FDA) announced that the unified Human Foods Program,…

On Oct. 1, 2024, the U.S. Food and Drug Administration (FDA) announced that the unified Human Foods Program,…

On Sept. 30, 2024, Amneal Pharmaceuticals announced that it had launched CREXONT ® (carbidopa and levodopa) extended-release capsules…

On Sept. 26, 2024, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers Squibb’s Cobenfy (xanomeline and trospium…

On Sept. 20, 2024, the U.S. Food and Drug Administration approved FluMist for self- or caregiver-administration. FluMist is…

On Sept. 13, 2024, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Ocrevus Zunovo™…

On Sep. 12, 2024, the the U.S. Food and Drug Administration (FDA) authorized the first over-the-counter (OTC) hearing…

On Aug. 30, 2024, Novavax announced the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) had received Emergency Use…

On Aug. 7, 2024, Amneal Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had approved CREXONT…
On Aug. 6, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved vorasidenib (Voranigo, Servier…
On Aug. 2, 2024, the U.S. Food and Drug Administration approved Adaptimmune’s Tecelra (afamitresgene autoleucel), a gene therapy…
On Jun. 27, 2024, the U.S. Food and Drug Administration granted marketing authorization to Cepheid for the Xpert…

On Jun. 20, 2024, the U.S. Food and Drug Administration expanded the approval of Sarepta Therapeutics’ Elevidys (delandistrogene…

On Jun. 17, 2024, Merck announced that the U.S. Food and Drug Administration (FDA) had approved CAPVAXIVE™ (Pneumococcal…
On Jun. 14, 2024, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously voted to recommend…

On Jun. 10, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On June 5, 2024, the U.S. FDA’s Vaccines and Related Biological Products Advisory Committee announced that it had…

On May 31, 2024, Moderna announced that the U.S. Food and Drug Administration (FDA) had approved mRESVIA (mRNA-1345),…

On May 14, 2024, Roche announced the U.S. FDA approval of its human papillomavirus (HPV) self-collection solution, one…

On Apr. 26, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BEQVEZ™ (fidanacogene…
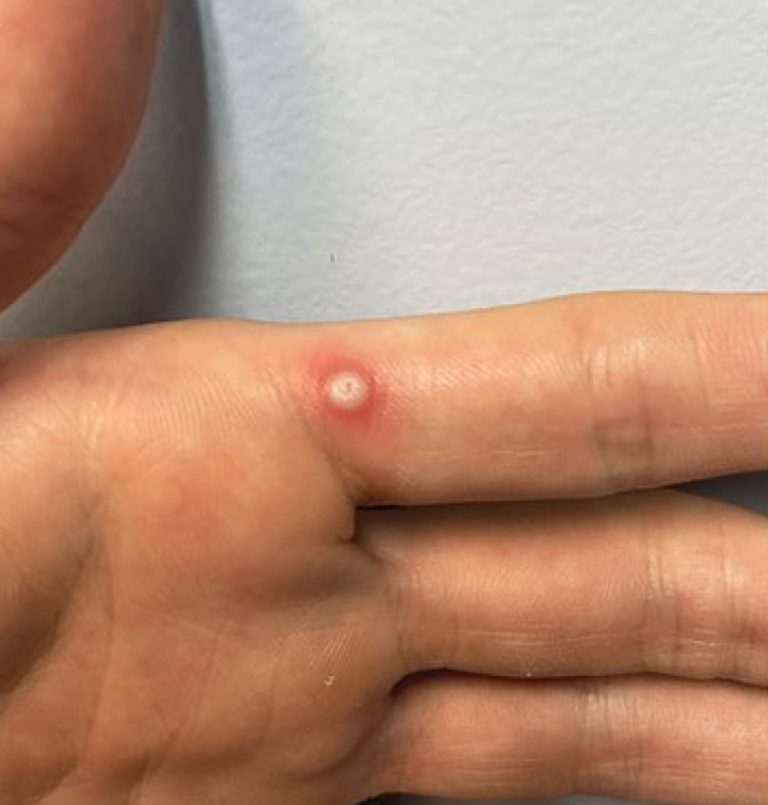
On Apr. 23, 2024, the U.S. Food and Drug Administration (FDA) announced that nearly all (99%) of the…
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Apr. 1, 2024, Otsuka Pharmaceutica and Click Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…

On Mar. 22, 2024, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Pemgarda…

On Mar. 21, 2024, the U.S. Food and Drug Administration (FDA) approved Italfarmaco’s Duvyzat (givinostat) oral medication for…

On Mar. 18, 2024, the U.S. Food and Drug Administration (FDA) approved Orchard Therapeutics’ Lenmeldy (atidarsagene autotemcel), the…
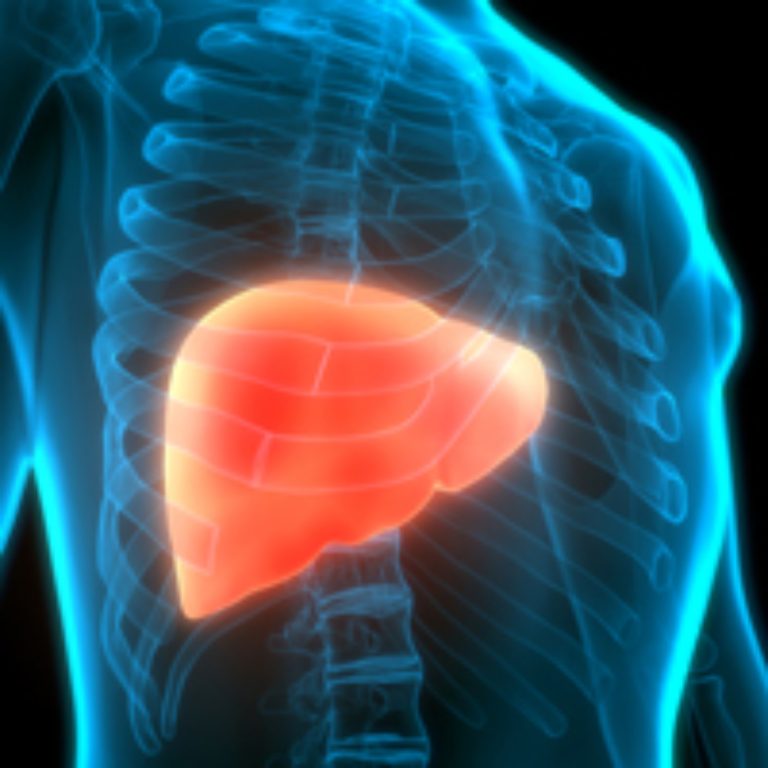
On Mar. 14, 2024, the U.S. Food and Drug Administration (FDA) approved Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) for the…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…