FDA approves ODACTRA® for the treatment of house dust mite allergy in young children
On Feb. 27, 2025, ALK announced that the U.S. Food and Drug Administration (FDA) has approved ALK’s ODACTRA®…

On Feb. 27, 2025, ALK announced that the U.S. Food and Drug Administration (FDA) has approved ALK’s ODACTRA®…

On Feb. 18, 2025, Hologic announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance…
On Feb. 12, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved a New…
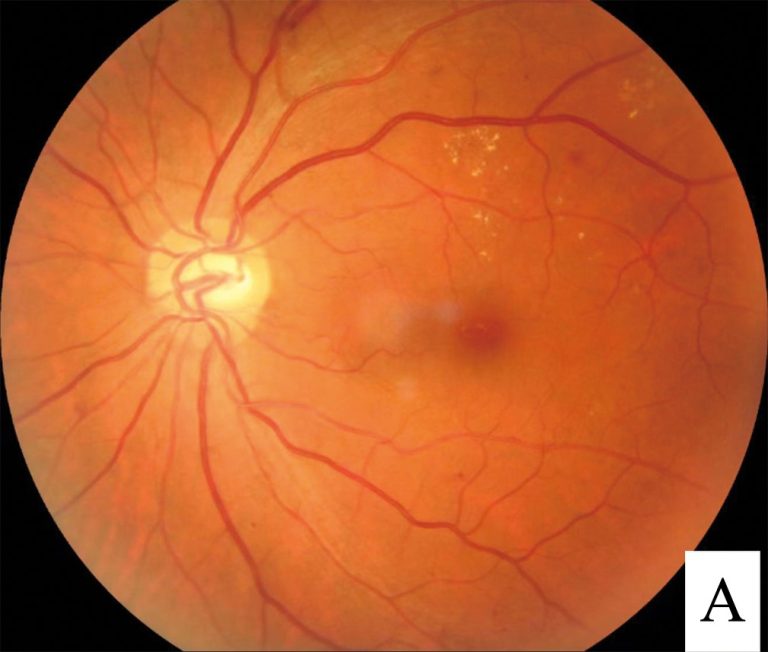
On Feb. 4, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Susvimo® (ranibizumab…

On Jan. 30, 2025, the U.S. Food and Drug Administration (FDA) approved Vertex Pharmaceuticals’s Journavx (suzetrigine) 50 milligram…

On Jan. 28, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has approved Ozempic® to…
Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…

On Jan. 15, 2025, the U.S. Food and Drug Administration (FDA) announced revoking the authorization for the use…

On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…

On Jan. 6, 2025, the U.S. Food and Drug Administration (FDA) issued guidance for industry on the action…

On Dec. 20, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved BRAFTOVI® (encorafenib)…

On Dec. 20, 2024, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) had approved Alhemo®…

On Dec. 19, 2024, the U.S. Food and Drug Administration announced it had updated the definition of “healthy…

On Dec. 18, 2024, the National Academies of Sciences released a report showing the current information ecosystem makes…

On Dec. 11, 2024 the Board of Directors of AquaBounty Technologies announced that the Company was winding down…
On Dec. 4, 2024, The U.S. Food and Drug Administration (FDA) announced approved of AstraZeneca’s blockbuster drug Imfinzi…
On Nov. 21, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that by the week…

On Nov. 15, 2024, the White House Office of Science and Technology Policy released a report on Building…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…

On Nov. 13, 2024, the U.S. Food and Drug Administration (FDA) and the U.S. Centers for Disease Control…
On Nov. 4, 2024, the U.S. Food and Drug Administration (FDA), in collaboration with the Environmental Protection Agency…

On Oct. 28, 2024, HOPO Therapeutics announced that it has been awarded a contract valued at up to…
On Oct. 22, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO® (Respiratory…

On Oct. 16, 2024, The U.S. Food and Drug Administration (FDA) announced it had updated the label for…

On Oct. 14, 2024, RedHill Biopharma announced that the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA)…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…
On Oct. 10, 2024, Genentech announced today that the U.S. Food and Drug Administration (FDA) approved ItovebiTM (inavolisib),…

On Oct. 8, 2024, Okanagan Specialty Fruits® (OSF), the developer and grower behind the innovative Arctic® apple varieties, announced that its…

On Oct. 4, 2024, Exact Sciences announced the U.S. Food and Drug Administration (FDA) had approved the Cologuard…

On Oct. 2, 2024, a study from the GBD Tobacco Forecasting Collaborators reported that by accelerating the decline…