Washington University’s Jeffrey T. Fort Neuroscience Research building dedicated
On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…

On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…
On Jan. 24, 2024, Oregon Health & Science University announced that Pamela Runner, 77, of Corvallis, became the…

On Jan. 10, 2024, an National Institutes of Health (NIH) supported study announced an n atlas revealing the…

On Jan. 4, 2024, France announced it had detected bird flu on a duck farm in the Vendee…
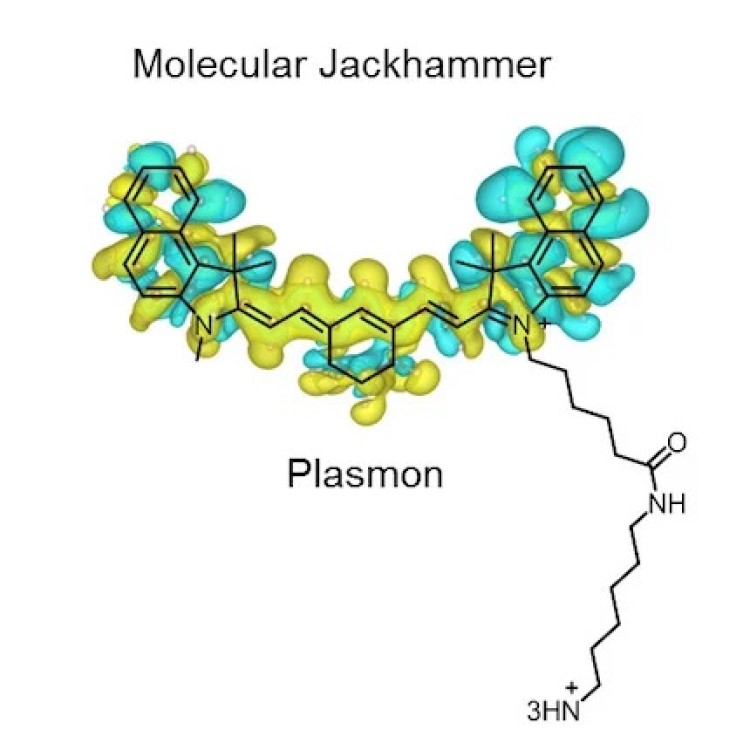
On Dec. 19, 2023, a research team led by Rice University reported they found that the atoms of…

On Dec. 18, 2023, the Novo Nordisk Foundation announced it was committing up to DKK 1.8 billion (USD…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Dec. 14, 2023, the University of Alberta announced that a study published in Nature sheds light on…
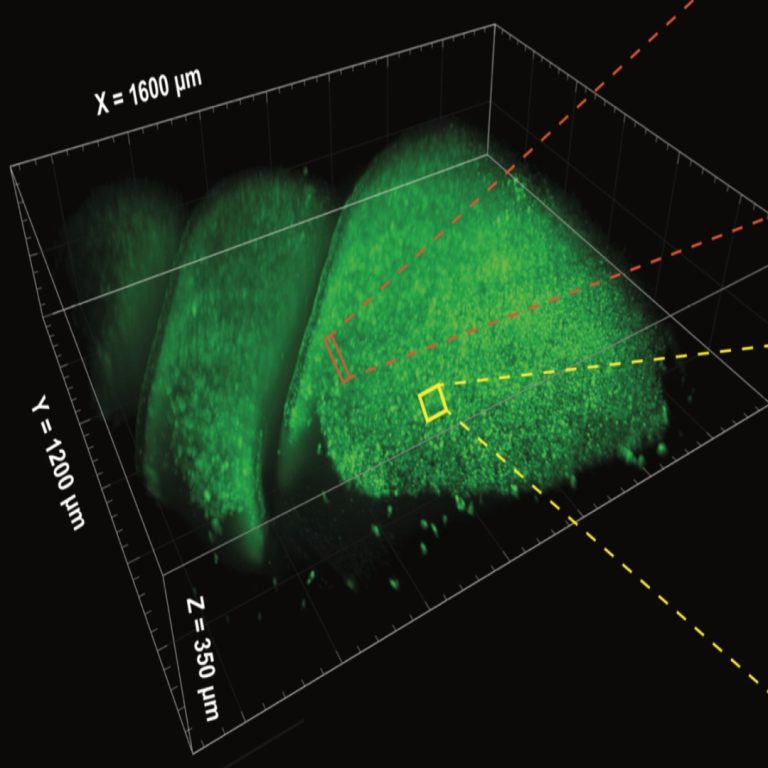
On Dec. 13, 2023, for the first time ever, an international team of researchers announced they had created…

On Dec. 13, 2023, National Institute of Allergy and Infectious Diseases (NIAID) scientists announced findings published in the…

On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…

On Nov. 30, 2023, in a momentous landmark for medical research, UK Biobank announced that it had unveiled…

On Nov. 29, 2023, the BC Centre for Disease Controlr Disease Control (BCCDC) announced that a A passenger…

On Nov. 17, 2023, the Oregon Department of Agriculture has new confirmed cases of avian influenza, and the…

On Nov. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov. 14, 2023, Yellowstone National Park and the Wyoming Game and Fish Department (WGFD) officials confirmed the…

On Nov. 13, 2023, the World Health Organization (WHO announced that the Ministry of Health and Social Welfare…
On Nov. 10, 2023, the Centers for Disease Control and Prevention (CDC) estimated that from October 1, 2023,…
On Nov. 9, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC)…
On Oct. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $42.1 million to fund various translational…

On Oct. 25, 2023, Bavarian Nordic announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory…

On Oct. 25, 2023, University of British Columbia researchers announced they had identified buried kimberlite, the rocky home…

On Oct. 23, 2023, the British Antarctic Survey (BAS) reported that Highly Pathogenic Avian Influenza (HPAI) had been…

On Oct. 20, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that among children and…

On Oct. 17, 2023, the U.S. Department of Agriculture’s (USDA) announced a new partnership between the National Bio…

On Oct. 14, 2023, Purdue University ensured that the memory of the former graduate and devoted Boilermaker football…

On Oct. 12, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that two fatal human…

On Oct. 11, 2023, the European Commission, the European Investment Bank and the Bill & Melinda Gates Foundation…

On Oct. 2, 2023, France began vaccinating ducks against bird flu to try and stem the virus that…