NEJM published phase 2b clinical trial results demonstrating efficacy of COVID-19 vaccine against B.1.351 variant
On May 5, 2021, Novavax announced the publication of results from the initial primary analysis of a Phase…

On May 5, 2021, Novavax announced the publication of results from the initial primary analysis of a Phase…

On May 5, 2021, Pfizer and BioNTech announced that Health Canada had expanded the Interim Order authorization for…

On May 5, 2021, CytoDyn announced an agreement to partner with the Albert Einstein Israelite Hospital (AEIH) in…

On May 4, 2021, Moderna announced an expansion of the Moderna Technology Center (MTC) in Norwood, MA including…

On May 3, 2021, the National Institutes of Health announced that researchers wre able to wirelessly record the…
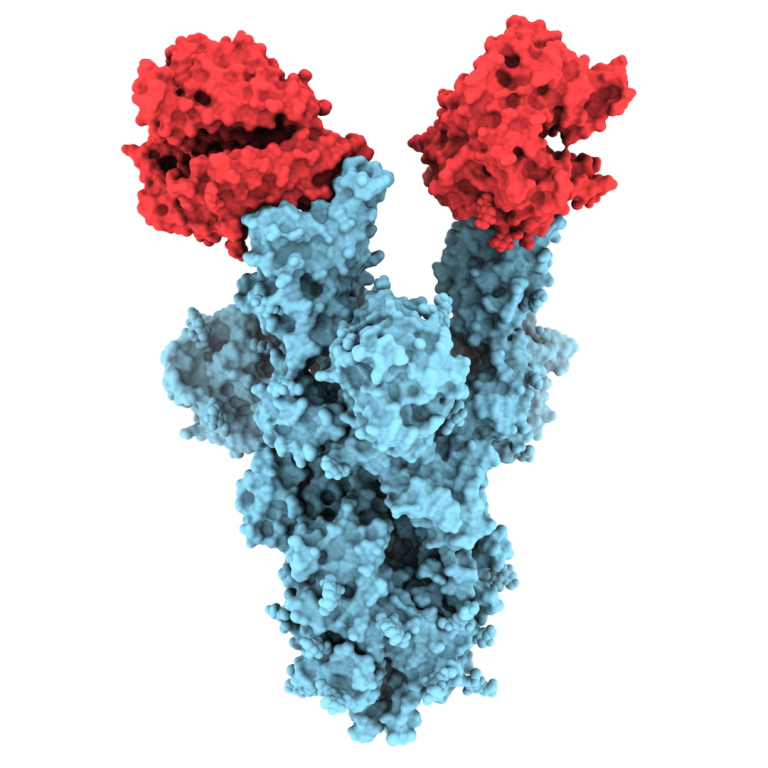
On May 3, 2021, University of British Columbia (UBC) researchers were the first in the world to publish…

On May 3, 2021, Precipio announced that it had successfully launched its COVID-19 rapid antibody test (20 minute)…

On May 3, 2021, Moderna announced an agreement with Gavi, the Vaccine Alliance to supply up to 500…

On May 3, 2021, Novavax announced that it had initiated a pediatric expansion of its Phase 3 clinical…

On May 3, 2021, Gavi, the Vaccine Alliance, that it had signed an advance purchase agreement with Moderna…

On Apr. 30, 2021, Moderna announced that the World Health Organization (WHO) had issued Emergency Use Listing (EUL)…

On Apr. 29, 2021, Lonza announced the expansion of its collaboration with Moderna to manufacture the drug substance…
On Apr. 29, 2021, researchers at the University of British Columbia (UBC) announced a study published in Cell…

On Apr. 29, 2021, scientists from several hospitals and research centers reported what happens in individual cells of…

On Apr. 29, 2021, Moderna announced it was making new funding commitments to increase supply at its owned…
On Apr. 29, 2021, Pfizer and BioNTech announced they had submitted a variation to the Conditional Marketing Authorization…

On Apr. 28, 2021, Rockefeller University researchers announced the launch the Vertebrate Genomes Project, an ambitious initiative launched…

COVID-19 and it’s naysayers are attacking Science and Reason. The defenders must suffer the slings and arrows from…

On Apr. 26, 2021, Moderna announced that it had entered into an agreement with Sanofi for fill/finish sterile…

On Apr. 23, 2021, Inovio Pharma announced that it was planning for a predominantly ex-U.S. Phase 3 trial…

On Apr. 20, 2021, Moderna announced a new supply agreement with Israel for 2022 whereby Israel also retained…

On Apr. 19, 2021, Pfizer and BioNTech announced supplying an additional 100 million doses of COMIRNATY, the companies…

On Apr. 19, 2021, the Broad Institute of MIT in partnership with the Centers for Disease Control and…

On Apr. 19, 2021, the Mayo Clinic announced a study that bolstered evidence that colorectal cancer is often…

On Apr. 19, 2021, Tonix Pharmaceuticals and OyaGen announced an exclusive worldwide licensing agreement for an antiviral inhibitor…

On Apr. 19, 2021, scientists at the Texas A&M University Global Health Research Complex (GHRC) announced they had…

On Apr. 19, 2021, CytoDyn announced it had submitted the manufacturing section (CMC) of the application for an…
On Apr. 15. 2021, a team of researchers led by the University of Minnesota Medical School announced they…

On Apr. 15, 2021, Merck announced the discontinuation of development of MK-7110 (formerly known as CD24Fc) for the…
On Apr. 15, 2021, researchers at the University of Oxford reported that the risk of the rare blood…