National Institutes of Health clinical trial of universal flu vaccine candidate began
On Sept. 15, 2023, researchers at the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…

On Sept. 15, 2023, researchers at the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…

On Sept. 14, 2023, the World Health Organization (WHO) reported that as of September 11, 2023, a total…

On Sept. 14, 2023, Northwestern University announced it had been awarded $50 million over five years from the…

On Sept. 12, 2023, the National Institutes of Health announced it was establishing the Multi-Omics for Health and…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Sept. 11, 2023, researchers at the National Institutes of Health (NIH) reported that living in an area…
![European Commission expanded Merck’s ERVEBO [Ebola Zaire vaccine] indication to include children 1 year of age and older](https://lifesciencehistory.com/wp-content/uploads/2022/08/european_commission_logo-768x768.jpg)
On Sept. 7, 2023, Merck announced that the European Commission (EC) had approved an expanded indication for ERVEBO…

On Sept. 6, 2023, the Markey Cancer Center (MCC) became Kentucky’s first and only National Cancer Institute (NCI)-Designated…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…

On Sept. 1, 2023, the Washington State Department of Health announced an outbreak of avian influenza, also known…

On Sept. 1, 2023, the North Dakota Development Fund, an investment vehicle for the North Dakota Department of…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Aug. 27, 2003, The Institute for Collaborative Biotechnologies (ICB) announced it was funding a $50 million five…

On Aug. 25. 2023, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Aug. 25, 2023, Harvardメs Center for Brain Science announced the ceation of an eye atlas that pinpoints…
On Aug. 24, 2023, NIAID reported that emerging resistance to common malaria treatments in Uganda could be connected…
On Aug. 23, 2023, a gene variant found almost exclusively in the genomes of people of African ancestry…
On Aug. 23. 2023, researchers co-led by University of California, Santa Cruz Assistant Professor of Biomolecular Engineering Karen…
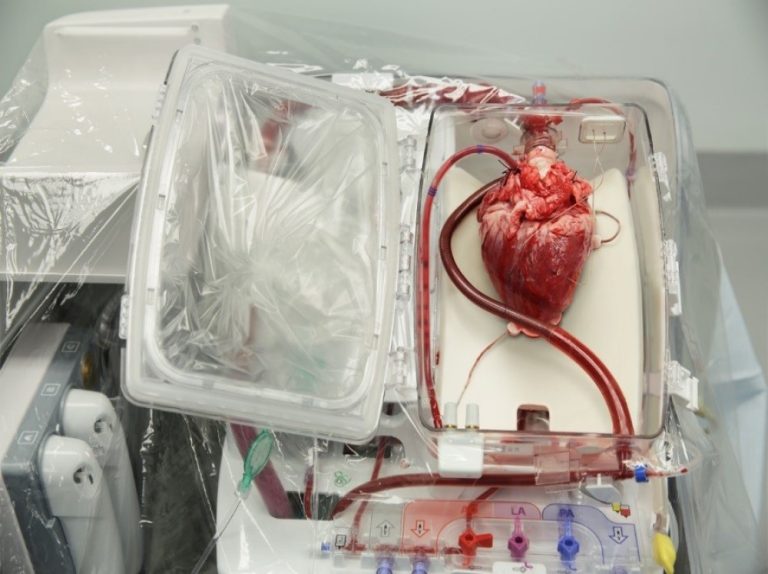
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…
On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…

On Aug. 22, 2023, Alberta Innovates announced that more than $13.6 million will go to 19 partners, across…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…

On Aug. 4, 2023, Biogen and Sage Therapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…

On Aug. 1, 2023, BD announced that the U.S. Food and Drug Administration (FDA) 510(k) had provided clearance…

On Aug. 1, 2023, the Finnish Food Authority specified the euthanasia criteria for fur animals infected with avian…