Nanoplastics can reduce the effectiveness of antibiotics
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…

On Oct. 29, 2024, a study led by Andrei Gudkov, PhD, DSci, at Roswell Park Comprehensive Cancer Center support…
On Oct. 29, 2024, the World Health Organization (WHO) published a report on tuberculosis revealing that approximately 8.2…
On Oct. 29, 2024, the World Health Organization (WHO) announced that it had, in collaboration with Member States,…
On Oct. 29, 2024, a study led by investigators from the UCLA Health Jonsson Comprehensive Cancer Center (UCLA…
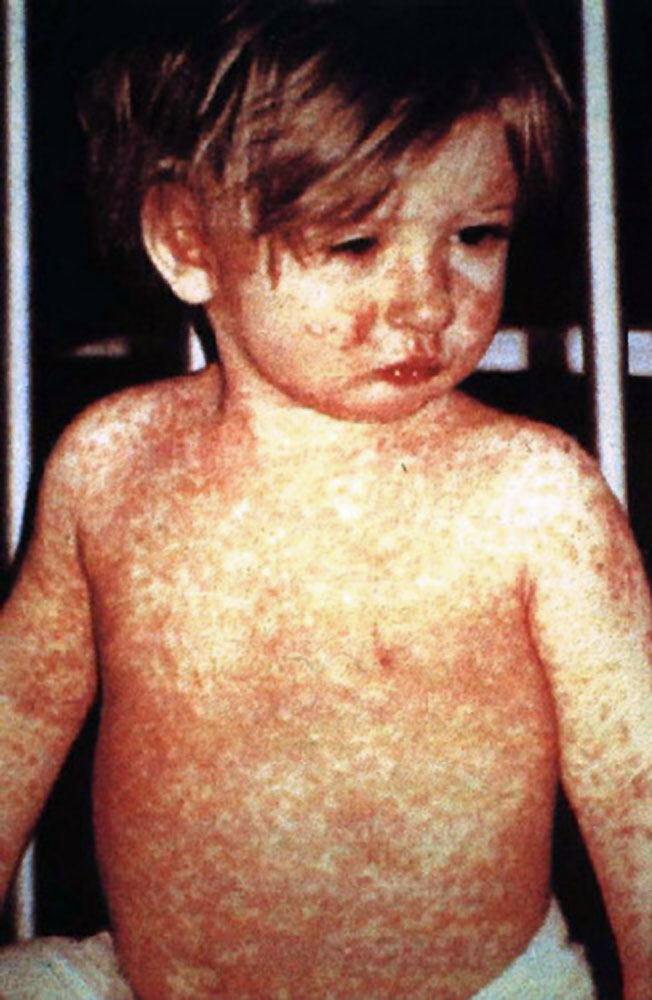
On Oct. 29, 2024, the BC Centre for Disease Control announced that travellers were alerted to potential exposure…

On Oct. 28, 2024, the Johns Hopkins University and Johns Hopkins Medicine, together with descendants of Henrietta Lacks,…

On Oct. 28, 2024, HOPO Therapeutics announced that it has been awarded a contract valued at up to…
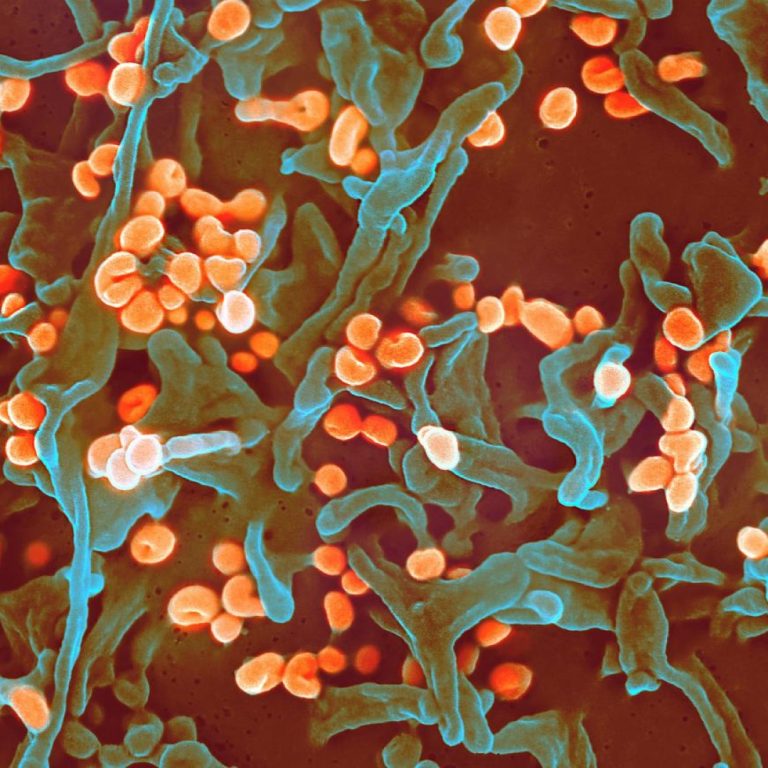
On Oct. 28, 2024, the Iowa Department of Health and Human Services (HHS) confirmed the death of a…
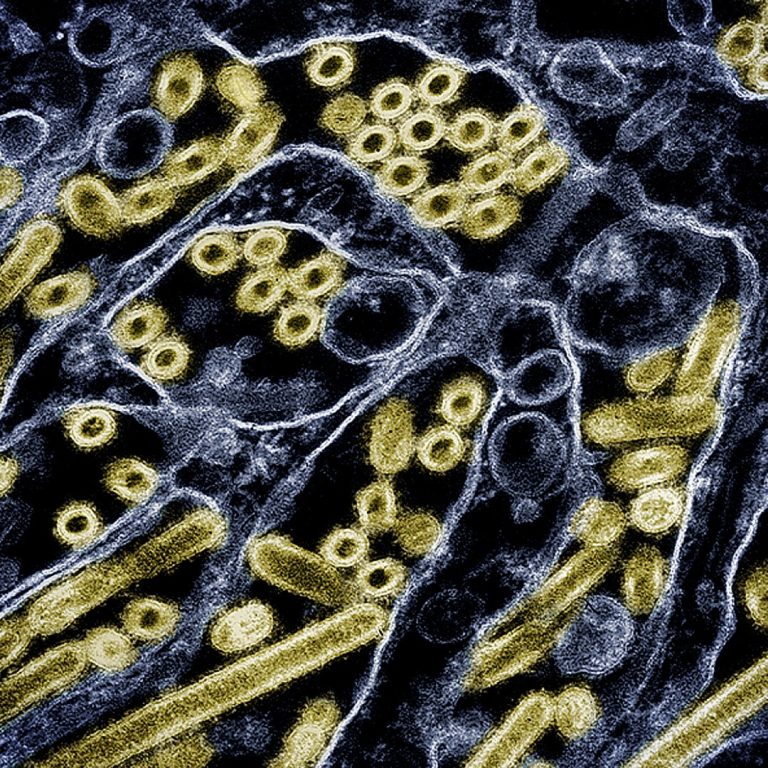
On Oct. 28, 2024, University of Wisconsin–Madison researchers reported that a strain of H5N1 avian influenza virus found…

On Oct. 25, 2024, the World Health Organization reported that as of October 24, a total of 64…

On Oct. 24, 2024, the Centers for Disease Control and Prevention (CDC) confirmed that two people who had…

On Oct. 24, 2024, researchers from Children’s Medical Center Research Institute at UT Southwestern reported that ancient viral remnants…

On Oct. 23, 2024, Pfizer announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…

On Oct. 23, 2024, the University of Rochester announced that the Saunders Foundation, led by University Trustee Emeritus…
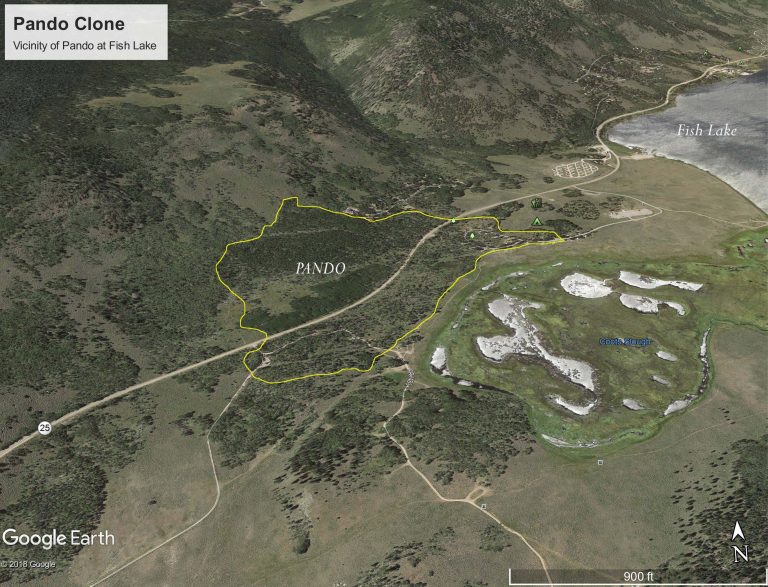
On Oct. 22, 2024, Utah State University researchers Paul Rogers and Darren McAvoy announced they have conducted the…
On Oct. 22, 2024, the U.S. Centers for Disease Control (CDC) announced that public health officials in multiple…

On Oct. 22, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO® (Respiratory…

On Oct. 21, 2024, scientists from the University of Alabama at Birmingham (UAB) and Iowa State University (ISU)…
On Oct. 21, 2024, in one of the largest-ever studies of DNA and brain volume, researchers announced they…
On Oct. 20, 2024, the World Health Organization (WHO) announced it had certified Egypt as malaria-free, marking a…

On Oct. 20, 2024, the Washington State Department of Health announced that four agricultural workers tested positive for…

On Oct. 18, 2024, a study published in JAMA Ophthalmology, found that approximately 4.22 million people in the…

On Oct. 18, 2024, the World Health Organization (WHO) reported that from January 1 to September 29, 2024,…

On Oct. 18, 2024, the California Department of Public Health (CDPH) reported that a total of 13 human…

On Oct. 18, 2024, a research team led by Radboud university medical center reported that a new PET…

On Oct. 17, 2024, Colossal Biosciences, an American biotechnology and genetic engineering company, announced that it has a…
On Oct. 17, 2024, Geisinger announced results of a study that showed increased risk for autism appears to…

On Oct. 17, 2024, a study led by the University at Buffalo and the Jackson Laboratory (JAX) revealed…

On Oct. 16, 2024, The U.S. Food and Drug Administration (FDA) announced it had updated the label for…