Roche announced U.S. FDA approval of Xofluza to treat influenza in children aged five years and older
On Aug. 11, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…

On Aug. 11, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…
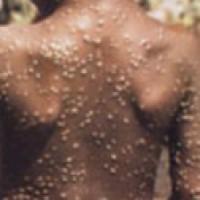
On Aug. 9, 2022, the U.S. Food and Drug Administration issued an emergency use authorization for the JYNNEOS…

On Aug. 9, 2022, Moderna announced an amendment to its agreement with the European Commission (EC) to convert…
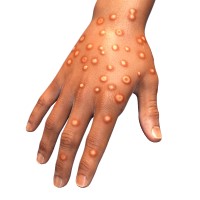
On Aug. 9, 2022, the the Department of Health and Human Services (HHS) announced it had determined that…

On Aug. 5, 2022, the Centers for Disease Control and Prevention (CDC) reported the first human infection with…

On Aug. 5, 2022, researchers from the National Institutes of Health announced that they had developed a three-dimensional…
On Aug. 5, 2022, the U.S. Food and Drug Administration (FDA) approved Daiichi Sankyo’s Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV…

On Aug. 4, 2022, Novavax announced the initiation of its Phase 2b/3 Hummingbird global clinical trial. The trial…
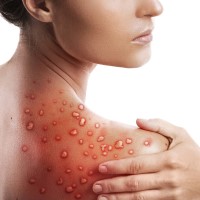
On Aug. 4, 2022, the U.S. Department of Health and Human Services declared the ongoing spread of monkeypox…

On Jul. 31, 2022, the National Aeronautics and Space Administration (NASA) paid respects to actor Nichelle Nichols, who…

On Jul. 30, 2022, scientists announced they had sequenced the genome of a 6,000-year-old Citrullus seeds that revealed…

On Jul. 29, 2022, the U.S. Department of Health and Human Services (HHS), in collaboration with the U.S….

On Jul. 28, 2022, AlphaFold, in partnership with EMBL’s European Bioinformatics Institute (EMBL-EBI), announced they we’re releasing predicted…
On Jul. 28, 2022, the Allen Institute announced a newly released dataset of 1.2M cells from brain donors…
On Jul. 28, 2022, the World Health Organization (WHO) released new guidelines for the use of long-acting injectable…

On Jul. 27, 2022, Pfizer and BioNTech announced that the companies had initiated a randomized, active-controlled, observer-blind, Phase…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…

On Jul. 27, 2022, the Centers for Disease Control and Prevention (CDC) announced that it had identified for…

On Jul. 27, 2022, BD (Becton, Dickinson) announced their newly developed molecular polymerase chain reaction (PCR) test for…

On Jul. 26, 2022, Novavax announced that the Australian Therapeutic Goods Agency had granted expanded approval for provisional…

On Jul. 26, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had received expanded manufacturing and marketing approval…
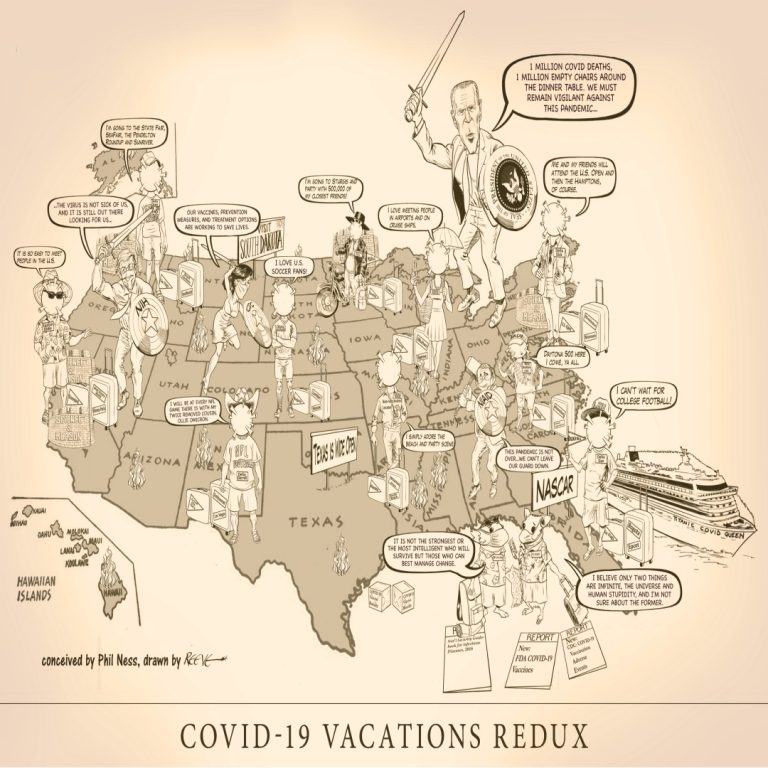
COVID-19 Vacations Redux iIlustrates once again the pits and perils vacation travelers face as they begin their Summer…

On Jul. 22, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On Jul. 22, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Jul. 22, 2022, a team of scientists announced they had sequenced the genome of a 6,000-year-old citrullus…
On Jul. 22, 2022, Gilead Sciences announced that the Committee for Medicinal Products for Human Use (CHMP) of…
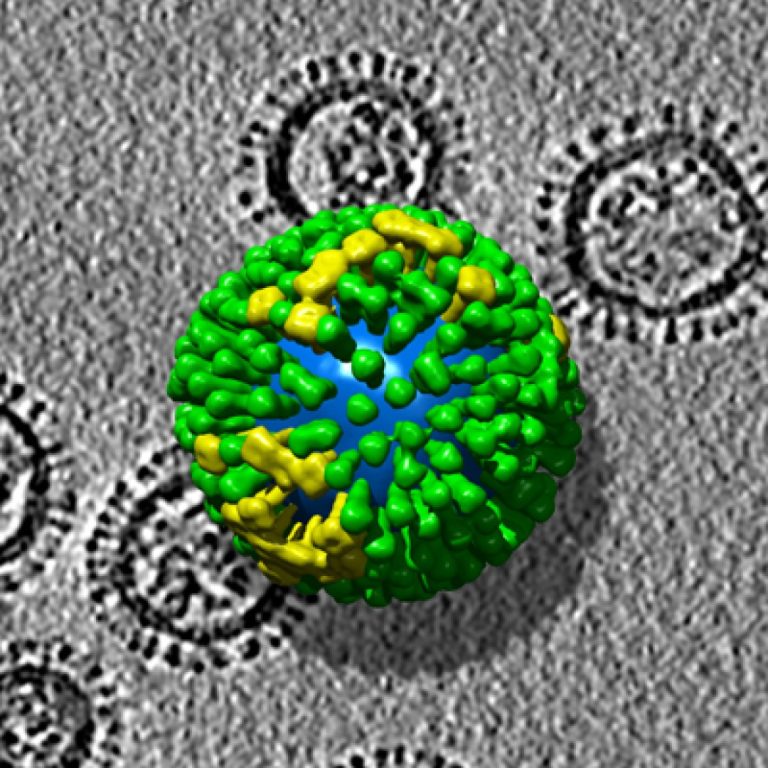
On Jul. 22, 2022, the Centers for Disease Control and Prevention (CDC) released a MMWR report that summarized…
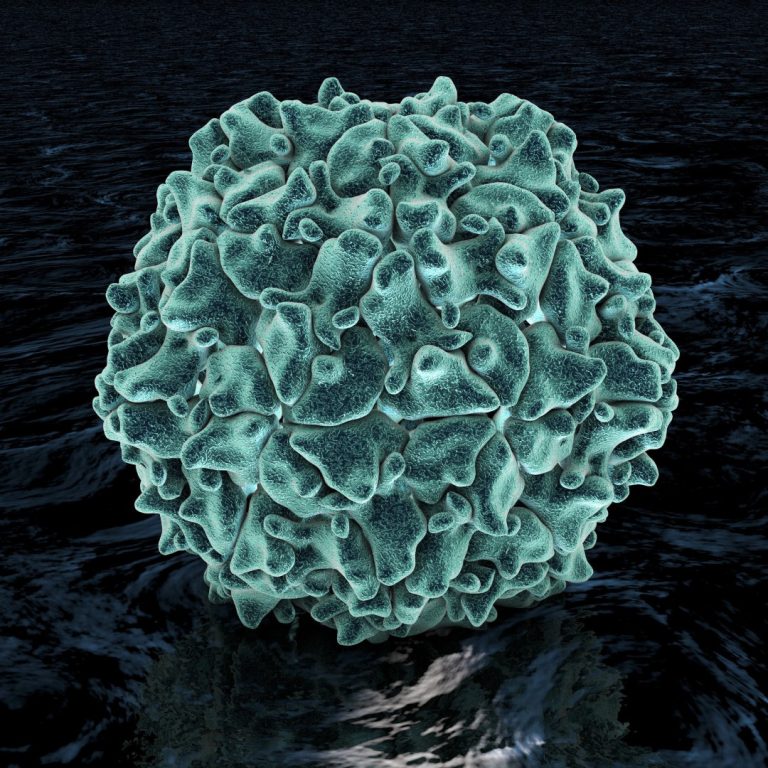
On Jul. 21, 2022, The New York State Department of Health confirmed that a case of paralytic poliomyelitis…
On Jul. 19, 2022, the NIH announced that although COVID-19 booster vaccinations in adults elicit high levels of…
On Jul. 19, 2022, Gilead Sciences and the European Commission announced a new joint procurement agreement (JPA) that…