CDC awarded $279 million to 49 States, DC, and 40 local health departments to help prevent drug overdoses
On Sept. 1, 2023, the U.S. Centers for Disease Control and Prevention (CDC) awarded $279 million to 49…

On Sept. 1, 2023, the U.S. Centers for Disease Control and Prevention (CDC) awarded $279 million to 49…

On Sept. 1, 2023, the North Dakota Development Fund, an investment vehicle for the North Dakota Department of…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Aug. 31, 2023, study results published in JAMA found that psilocybin treatment was associated with a clinically…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…
On Aug. 30, 2023, Emergent BioSolutions announced NARCANᆴ Naloxone HCl Nasal Spray 4 mg would be available on…

On Aug. 29, 2023, the COVID-19 Technology Access Pool (C-TAP), a multi-stakeholder partnership to facilitate sharing of intellectual…

On Aug. 28, 2023, in a study of 152 deceased athletes less than 30 years old who were…

On Aug. 27, 2003, The Institute for Collaborative Biotechnologies (ICB) announced it was funding a $50 million five…

On Aug. 25. 2023, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Aug. 25, 2023, Harvardメs Center for Brain Science announced the ceation of an eye atlas that pinpoints…
On Aug. 24, 2023, the U.S. Food and Drug Administration approved Sandoz’s Tyruko (natalizumab-sztn), the first biosimilar to…

On Aug. 24, 2023, researchers at City of Hope announced were awarded $32.3 million from the California Institute…
On Aug. 24, 2023, NIAID reported that emerging resistance to common malaria treatments in Uganda could be connected…

On Aug. 23, 2023, the CDC announced it had detected a new SARS-CoV-2 variant labeled BA.2.86. CDC was…
On Aug. 23. 2023, researchers co-led by University of California, Santa Cruz Assistant Professor of Biomolecular Engineering Karen…
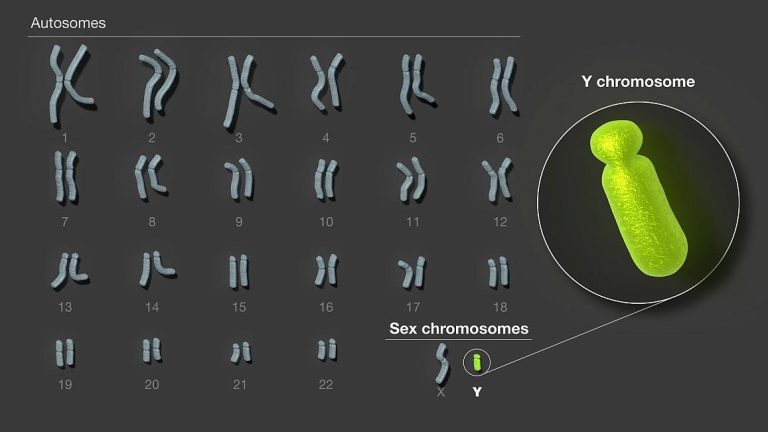
On Aug. 23, 2023, a gene variant found almost exclusively in the genomes of people of African ancestry…
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…
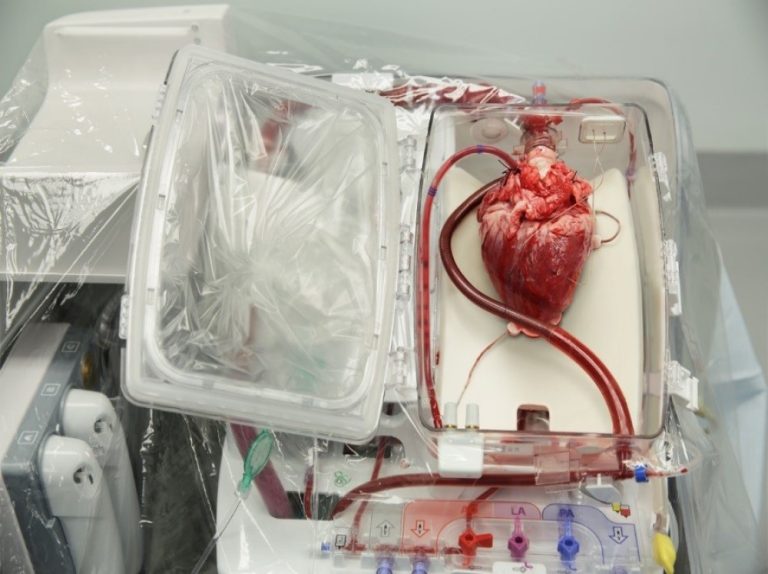
On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…

On Aug. 22, 2023, Alberta Innovates announced that more than $13.6 million will go to 19 partners, across…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 18, 2023, the Maryland Department of Health has confirmed and reported a positive case of locally…
On Aug. 17, 2023, the National Institutes of Health announced it had awarded $24 million in first-year funding…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…

On Aug. 16, 2023, surgeons at NYU Langone Health announed they had transplanted a genetically engineered pig kidney…

On Aug. 14, 2023, the National Institute of Health announced that it had released a comprehensive dataset that…
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…
On Aug. 9, 2023, the World Health Organization (WHO) released standing recommendations for battling COVID-19 and released an…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…