U.S. FDA approved first biosimilar to treat Multiple Sclerosis
On Aug. 24, 2023, the U.S. Food and Drug Administration approved Sandoz’s Tyruko (natalizumab-sztn), the first biosimilar to…
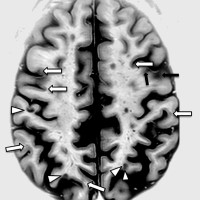
On Aug. 24, 2023, the U.S. Food and Drug Administration approved Sandoz’s Tyruko (natalizumab-sztn), the first biosimilar to…
On Aug. 23, 2023, a gene variant found almost exclusively in the genomes of people of African ancestry…

On Aug. 23, 2023, the CDC announced it had detected a new SARS-CoV-2 variant labeled BA.2.86. CDC was…
On Aug. 23. 2023, researchers co-led by University of California, Santa Cruz Assistant Professor of Biomolecular Engineering Karen…
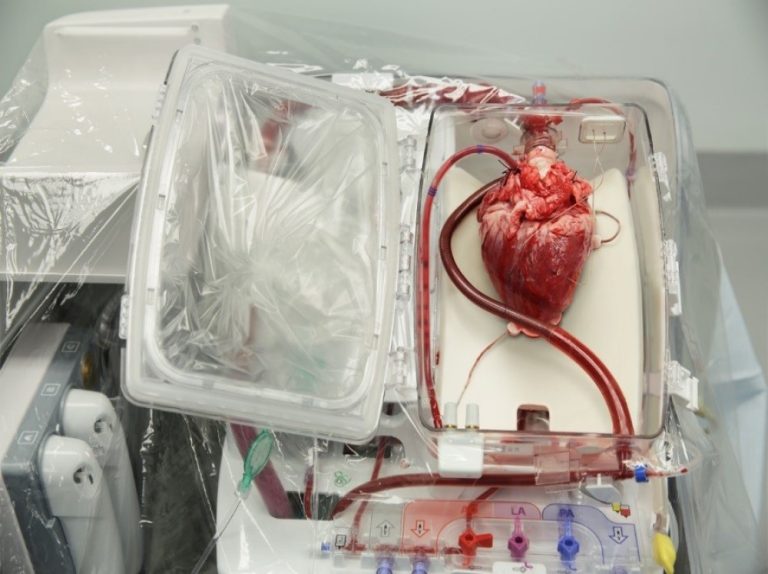
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…

On Aug. 22, 2023, Alberta Innovates announced that more than $13.6 million will go to 19 partners, across…

On Aug. 21, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory…

On Aug. 18, 2023, the Maryland Department of Health has confirmed and reported a positive case of locally…
On Aug. 17, 2023, the National Institutes of Health announced it had awarded $24 million in first-year funding…

On Aug. 16, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had approved over…

On Aug. 16, 2023, surgeons at NYU Langone Health announed they had transplanted a genetically engineered pig kidney…

On Aug. 14, 2023, the National Institute of Health announced that it had released a comprehensive dataset that…
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…
On Aug. 9, 2023, the World Health Organization (WHO) released standing recommendations for battling COVID-19 and released an…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Aug. 4, 2023, Biogen and Sage Therapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Aug. 4, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported the first two U.S….

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…

On Aug. 1, 2023, the Finnish Food Authority specified the euthanasia criteria for fur animals infected with avian…

On Aug. 1, 2023, the U.S. Dept of Agriculture (USDA) announced the groundbreaking of a new Plant Sciences…
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…

On Aug. 1, 2023, BD announced that the U.S. Food and Drug Administration (FDA) 510(k) had provided clearance…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
On Jul. 31, 2023, scientists at Washington University in St. Louis have developed a breath test that quickly…

On Jul. 31, 2023, the U.S. Department of Health and Human Services announced the formation of the Office…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…

On Jul. 28, 2023, in a first-of-its-kind clinical trial, bioelectronic medicine researchers, engineers and surgeons at Northwell Healthメs…
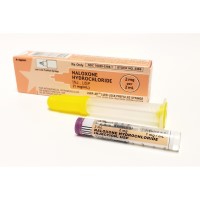
On Jul. 28, 2023, the U.S. FDA approved RiVive, 3 milligram (mg) naloxone hydrochloride nasal spray for over-the-counter…