CEPI and PATH strengthen partnership to accelerate development of vaccines against diseases with epidemic potential
On Dec. 14, 2023, The Coalition for Epidemic Preparedness Innovations (CEPI) and PATH today announced a renewed collaboration…

On Dec. 14, 2023, The Coalition for Epidemic Preparedness Innovations (CEPI) and PATH today announced a renewed collaboration…

On Dec. 14, 2023, Pfizer announced it had completed the acquisition of Seagen, a global biotechnology company based…

On Dec. 14, 2023, Biogen and Sage Therapeutics announced ZURZUVAE (zuranolone) 50 mg (two 25 mg capsules per…

On Dec. 13, 2023, National Institute of Allergy and Infectious Diseases (NIAID) scientists announced findings published in the…

On Dec. 13, 2023, in a study of historic scale, scientists at Gladstone Institutes announced that they had…
On Dec. 13, 2023, the Allen Institute and collaborators announced that after six years and 32 million cells,…
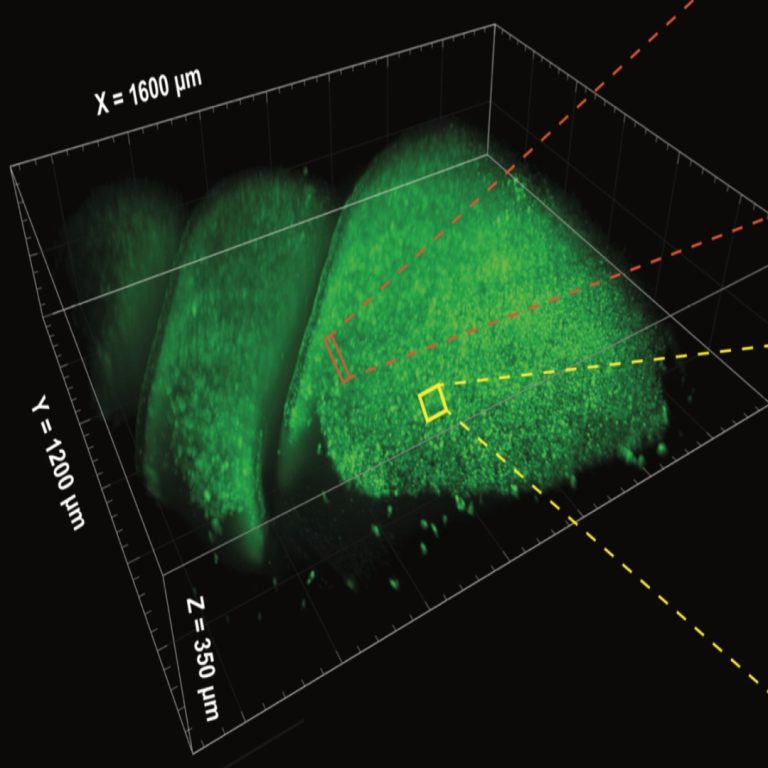
On Dec. 13, 2023, for the first time ever, an international team of researchers announced they had created…
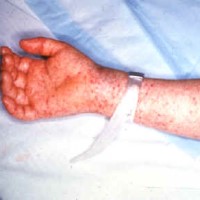
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, the U.S. Food and Drug Administration approved two milestone treatments, Casgevy and Lyfgenia, representing…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…

On Dec. 8, 2023, the University of Virginia broke ground Friday on the Paul and Diane Manning Institute…

On Dec. 7, 2023, the U.S. Food and Drug Administration reported that of the more than 116 medically…

On Dec. 7, 2023, BioNTech announced that it had signed a multi-year strategic partnership with the State of…

On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…

On Dec. 6, 2023, the federal government announced it had expanded the Home Test to Treat program, an…
On Dec. 6, 2023, the University of Missouri Research Reactor (MURR) announced they had completed its first commercial…

On Dec. 5, 2023, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid XBB.1.5 Vaccine (Recombinant…
On Dec. 5, 2023, Novartis announced that the U.S. Food and Drug Administration (FDA) had approved Fabhaltaᆴ (iptacopan)…

On Dec. 4 2023, the University of Wisconsin-Madison researchers announced that while analyzing data from wastewater samples collected…

On Dec. 3, 2023, the Bill & Melinda Gates Foundation announced that global donors at COP28 had pledged…
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…

On Nov. 30, 2023, researchers at Tufts University announced they had created tiny biological robots that they call…

On Nov. 30, 2023, in a momentous landmark for medical research, UK Biobank announced that it had unveiled…

On Nov. 30, 2023, the U.S. Department of Department of Agricultureメs (USDA) Animal and Plant Health Inspection Service…

On Nov. 30, 2023, researchers at Hokkaido University, in collaboration with colleagues at Woods Hole Oceanographic Institution announced…

On Nov. 29, 2023, Novavax’s partner, SK bioscience, announced that Novavax’s updated COVID-19 vaccine (NVX-CoV2601) had received Emergency…

On Nov. 29, 2023, the BC Centre for Disease Controlr Disease Control (BCCDC) announced that a A passenger…

On Nov. 29, 2023, the World Health Organization (WHO) announced that between 24 and 25 November 2023, the…

On Nov. 28, 2023, CSL and Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor and Welfare (MHLW)…