Genetic secrets from 4,000-year-old teeth illuminate the impact of changing human diets over the centuries
On Mar. 27, 2024, researchers from Trinity College Dublin announced they had recovered remarkably preserved microbiomes from two…
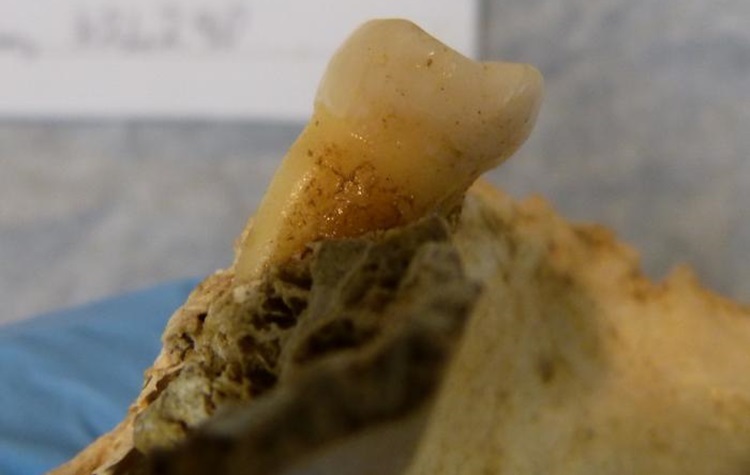
On Mar. 27, 2024, researchers from Trinity College Dublin announced they had recovered remarkably preserved microbiomes from two…
On Mar. 27, 2024, the World Health Organization announced it had launched a network for coronaviruses, CoViNet, to…
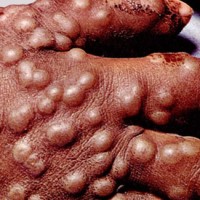
On Mar. 26, 2024, the National Academies of Sciences, Engineering, and Medicine released a report that said action…

On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…

On Mar. 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle…

On Mar. 22, 2024, the White House announced the creation of the National Bioeconomy Board. The Board will…

On Mar. 22, 2024, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Pemgarda…

On Mar. 21, 2024, Yield10 Bioscience announced that USDA-APHIS’s Biotechnology Regulatory Services (“BRS”) has determined that Yield10’s Camelina…
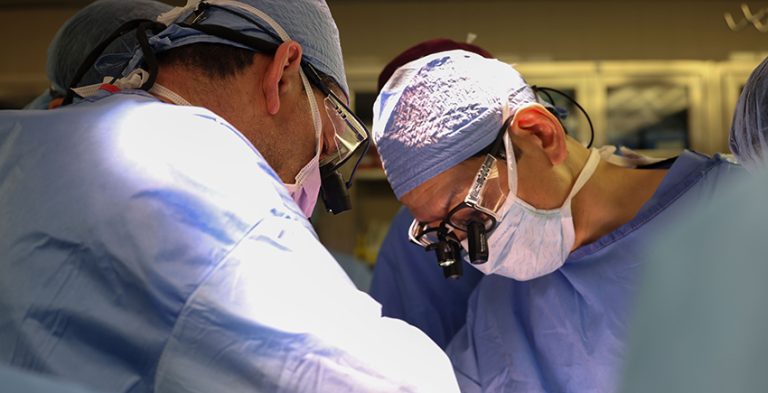
On Mar. 21, 2024, Harvard Medical School physician-scientists at Massachusetts General Hospital announced they had successfully transplanted a…

On Mar. 21, 2024, the U.S. Food and Drug Administration (FDA) approved Italfarmaco’s Duvyzat (givinostat) oral medication for…

On Mar. 21, 2024, Eledon Pharmaceuticals announced that tegoprubart, the company’s investigational anti-CD40L antibody, was used as a…

On Mar. 20, 2024, a National Institutes of Health-supported study reported that SARS-CoV-2, the virus that causes COVID-19,…

On Mar. 20, 2024, the U.S. Department of Agriculture (USDA) announced they had reviewed a University of Wisconsin…

On Mar. 20, 2024, the Minnesota Board of Health announced that a Stevens County goat kid (juvenile goat)…

On Mar. 19, 2024, scientists at the United States Department of Agriculture (USDA)メs Agricultural Research Service (ARS) announced…
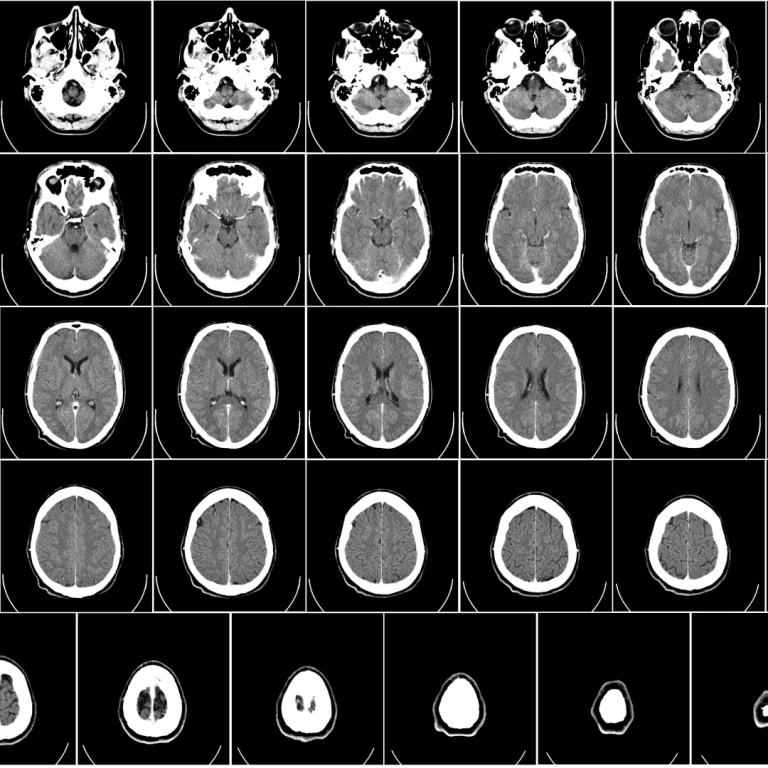
On Mar. 18, 2024, using advanced imaging techniques and in-depth clinical assessments, a research team at the National…

On Mar. 18, 2024, the U.S. Food and Drug Administration (FDA) approved Orchard Therapeutics’ Lenmeldy (atidarsagene autotemcel), the…

On Mar. 18, 2024, the U.S. Environmental Protection Agency (EPA) announced a final rule to prohibit ongoing uses…

On Mar. 14, 2024, Microsoft and the Fred Hutch announced a collaboration to end cancer faster, applying machine…
On Mar. 14, 2024, the Institute for Health Metrics and Evaluation (IHME) released all-cause mortality, life expectancy, and…
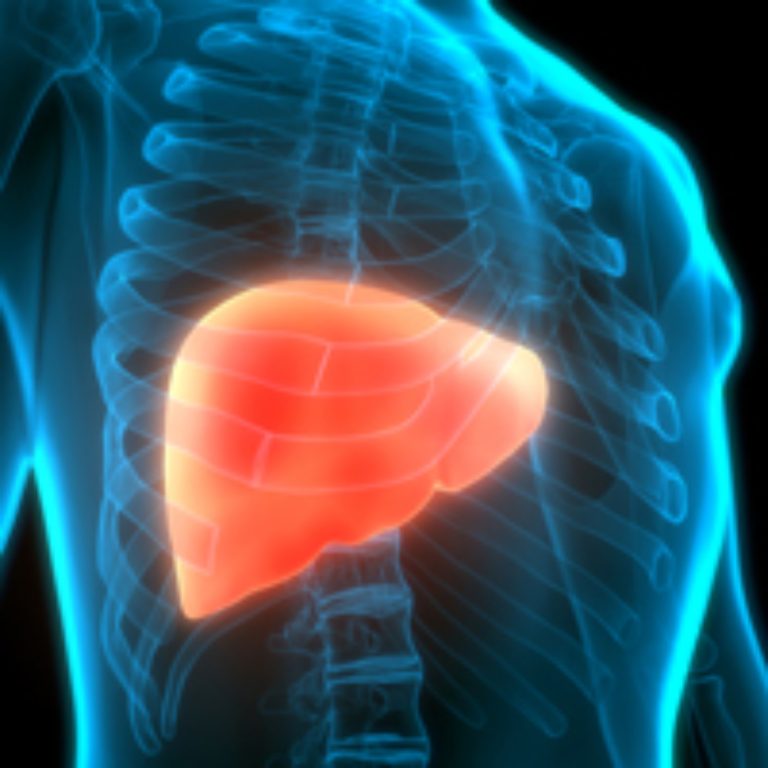
On Mar. 14, 2024, the U.S. Food and Drug Administration (FDA) approved Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) for the…

On Mar. 13, 2024, researchers at the National Institutes of Health (NIH) announced they had discovered that symptoms…

On Mar. 13, 2024, an unassuming brown bovine from the south of Brazil has made history as the…

On Mar. 13, 2024, the Fred Hutchinson Cancer Center announced that a blood test intended for screening for…

On Mar. 13, 2024, the National Science Board (NSB) published The State of U.S. Science and Engineering 2024,…
On Mar. 12, 2024, the United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) reported that the…

On Mar. 11, 2024, the British Antarctic Survey reported that penguins on the sub-Antarctic Islands of South Georgia…

On Mar. 8, 2024, the U.S. Food and Drug Administration approved a new indication for use of Novo…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…

On Mar. 5, 2024, the World Health Organization announced that In February 2024, Austria, Denmark, Germany, Sweden and…