California Department of Public Health Warned Against Drinking Second Lot of Raw Milk Following Bird Flu Detection
On Nov. 27, 2024, the California Department of Public Health (CDPH) issued a second warning to Californians to…

On Nov. 27, 2024, the California Department of Public Health (CDPH) issued a second warning to Californians to…
On Nov. 27, 2024, researchers at MUSC Hollings Cancer Center reported that cervical cancer deaths have plunged dramatically…

On Nov. 26, 2024, scientists at Texas A&M University announced they have found that healing the gut may…

On Nov. 26, 2024, the U.S. Department of Agriculture (USDA) announced it had temporarily paused imports of Mexican…
On Nov. 26, 2024, the the United Nations reported that fewer people contracted HIV last year than at…
On Nov. 25, 2024, the Texas Department of State Health Services reported that the first locally acquired case…

On Nov. 24, 2024, the California Department of Public Health (CDPH) issued a warning the public to avoid…

On Nov. 22, 2024, The Centers for Disease Control and Prevention (CDC) has confirmed a human infection with…

On Nov. 22, 2024, the Public Health Agency of Canada (PHAC) confirmed the first case of clade I…

On Nov. 22, 2024, the Chief Veterinary Officer of Mexico notified the U.S. Department of Agriculture’s (USDA) Animal…

On Nov. 21, 2024, teams at the University of Texas Southwestern Medical Center report a new way that…

On Nov. 21, 2024, a research team from China reported a fully phased genome assembly of ‘Fuji’ apple,…
On Nov. 21, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding $29.7 million to…

On Nov. 21, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that by the week…
On Nov. 21, 2024, the World Health Organization (WHO) reported that From January 1 to October 27, 2024,…

On Nov. 21, 2024, the U.S. Food and Drug Administration (FDA) announced that the investigation of a multistate…
On Nov. 21, 2024, a study from scientists at the UCLA Health Jonsson Comprehensive Cancer Center helps explain why…

On Nov. 21, 2024, scientists at the University of Florida (UF) announced they have developed a pioneering tool…

On Nov 20, 2024, researchers from the Wellcome Sanger Institute and collaborators have used cutting-edge genomic techniques to…
On Nov. 20, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov, 20, 2024, Pfizer announced that the European Commission (EC) has granted marketing authorization for HYMPAVZI™ (marstacimab)…

On Nov. 20, 2024, researchers with the global Human Cell Atlas (HCA) consortium reported significant progress in their…

On Nov. 20, 2024, a research collaboration between the Texas A&M College of Veterinary Medicine and Biomedical Sciences…

On Nov. 19, 2024, the California Department of Public Health (CDPH) reported it has identified a possible bird…
On Nov. 19, 2024, the World Health Organization (WHO) announced it had granted Emergency Use Listing (EUL) to Japan-based…
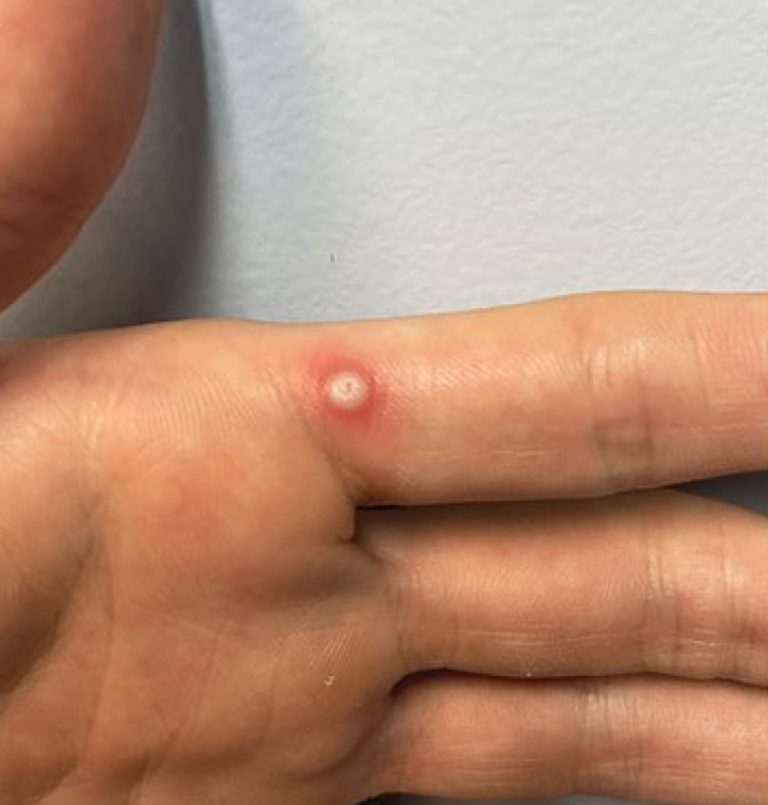
On Nov. 16, 2024, the California Department of Public Health (CDPH) announced it had identified through laboratory testing…
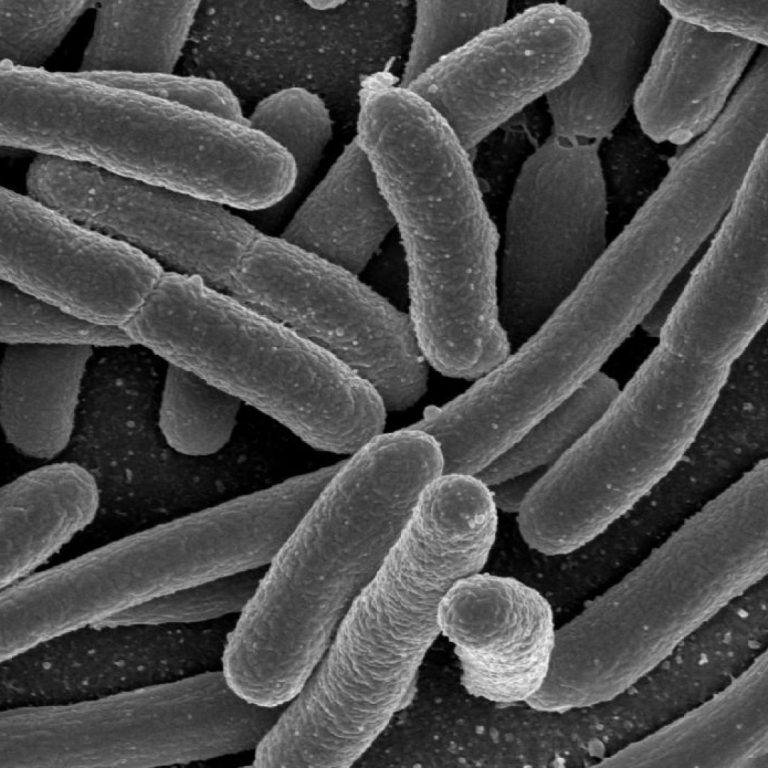
On Nov. 16, 2024, the U.S. Centers for Disease Control and Prevention (CDC) and public health officials in…
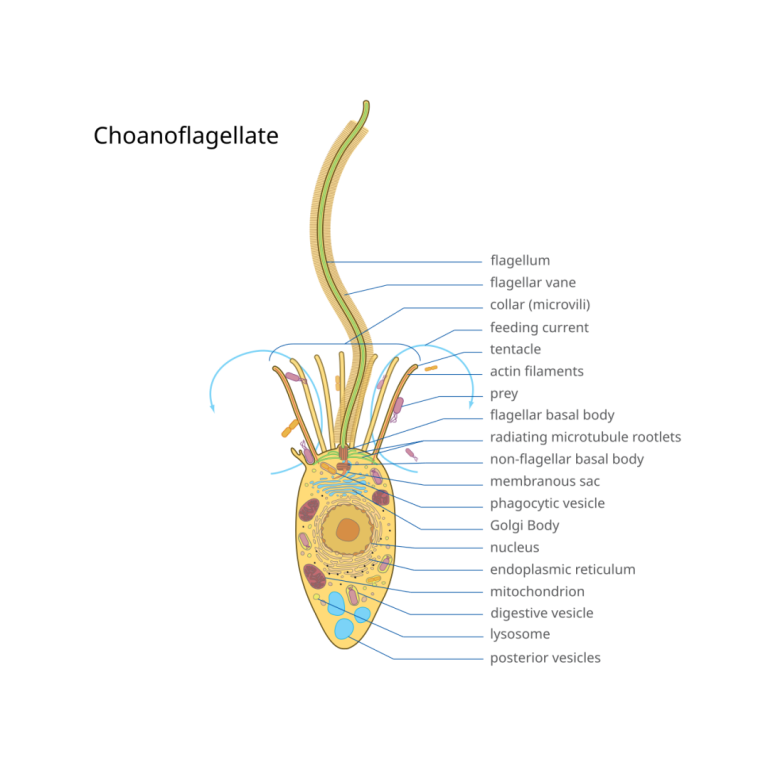
On Nov. 15, 2024, researchers from Queen Mary University of London and the the University of Hong Kong…

On Nov. 15, 2024, the White House Office of Science and Technology Policy released a report on Building…
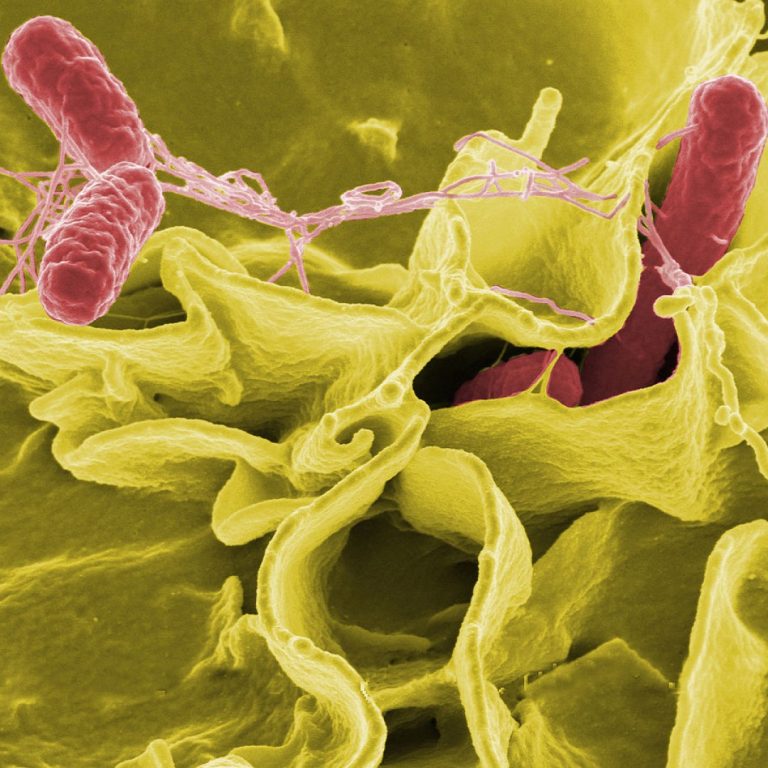
On Nov. 15, 2024, a University of California Davis Health (UC Davis Health) research team announced they had…